Comparative Prevalence of Antimicrobial Resistance in Community-Acquired Urinary Tract Infection Cases from Representative States of Northern and Southern India
Shivani Gupta1, Suman Kapur2, DV Padmavathi3
1 Research Scholar, Department of Biological Sciences, Birla Institute of Technology and Sciences (BITS Pilani), Hyderabad campus, Jawahar Nagar, Shameerpet Mandal, R.R. District, Hyderabad, India.
2 Professor, Department of Biological Sciences, Birla Institute of Technology and Sciences (BITS Pilani), Hyderabad campus, Jawahar Nagar, Shameerpet Mandal, R.R. District, Hyderabad, India.
3 Research Scholar, Department of Biological Sciences, Birla Institute of Technology and Sciences (BITS Pilani), Hyderabad campus, Jawahar Nagar, Shameerpet Mandal, R.R. District, Hyderabad, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Suman Kapur, Professor, Department of Biological Sciences, Birla Institute of Technology and Sciences (BITS Pilani), Hyderabad campus, Jawahar Nagar, Shameerpet Mandal, R.R. District, Hyderabad-500078, India. Phone : 09010202863, E-mail : skapur@hyderbad.bits-pilani.ac.in
Context: Urinary tract infections (UTIs) are amongst the most common infections described in outpatient settings. Increased antimicrobial resistance (AMR) of urinary tract pathogens is a matter of global public health concern. Treatment of UTI depends on both prevalence and antimicrobial resistance (AMR) of causative bacteria at any specific geographical location.
Aim: This study was undertaken to compare the prevalence of uropathogens and their AMR profile in two different geographical parts of India.
Materials and Methods: Clean-catch mid-stream urine samples were collected from adult patients, bacterial flora isolated from human urine was evaluated for antimicrobial susceptibility profile using Kirby Bauer’s disc diffusion method among patients from Hyderabad (Southern India), Rajasthan and Punjab (Northern India). The data were analysed using Chi-square (χ2) test, confidence interval (CI), odds ratio (OR) analysis and p-value using SPSS 16 software.
Results:Escherichia coli (55.1%) were the most prevalent isolates followed by Enterococcus faecalis (15.8%). Amikacin was the most active antimicrobial agents which showed low resistance rate of 14%. The present study revealed the geographical difference in prevalence of uropathogens with Klebsiella pneumoniae being the second most common uropathogen followed by E. faecalis in the states from northern India while no K. pneumoniae was seen in samples from southern India but E. faecalis was the second most prevalent organism.
Conclusion: Therefore, development of regional surveillance programs is highly recommended for implementation of national CA-UTI guidelines in Indian settings.
Antimicrobial resistance, Bacteriuria and antibiotics, Community-acquired urinary yract infections, Uropathogens
Introduction
Urinary tract infection (UTI) is the most common infectious disease after respiratory tract infection in community practice. It remains a major public health problem in terms of morbidity and financial cost with an estimated 150 million cases per annum worldwide, costing global economy in excess of six billion US dollars [1,2]. UTI is defined as bacteriuria along with urinary symptoms [3]. It may involve only the lower urinary tract or may involve both the upper and lower tract. Malnutrition, poor hygiene, low socio-economic status is important factors associated with UTIs [4].
The most episodes of UTI are caused by Escherichia coli (E.coli) and Enterococcus faecalis (E. faecalis), while Klebsiella pneumoniae (K. pneumoniae) accounts for most of the remaining infections [5]. Although E.coli has been reported as the commonest isolate causing UTI, recent reports suggest a changing pattern in the prevalence of uropathogens [6,7]. The introduction of antimicrobial therapy has contributed significantly to the management of UTIs along with other infectious diseases. In almost all cases of community-acquired UTI (CA-UTI), empirical antimicrobial treatment is initiated before the laboratory results for urine culture are available; contributing significantly to antimicrobial resistance (AMR) in uropathogens due to frequent and sometimes repeated misuse of antimicrobials [8]. The resistance pattern of community acquired uropathogens has not been extensively studied in the Indian subcontinent [9,10]. It is important to realize that there may be marked differences between various geographical areas. Since most UTIs are treated empirically the selection of antimicrobial agent should be determined not only by the most likely pathogen but also by its confirmed susceptibility pattern. Therefore, periodic monitoring of aetiological agents of UTI, and their resistance pattern in the community is essential for prudent empirical antibiotic therapy to control the menace of increasing AMR so as to maintain efficacy of available antibiotics. It was against this backdrop that the current study was undertaken to assess and compare the most frequent pathogens responsible for UTIs in outpatients and their AMR pattern in Southern and Northern Indian states. Additionally, the study also aimed at identifying the possible resistance trends.
Materials and Methods
Study area and study population
A retrospective study of all pathogens isolated from urine specimens of patients (both male and female; age 14-72 y) who attended the outpatient departments (OPDs) during the period January 2010 to June 2011 in Birla Sarvajanik hospital, Pilani (Rajasthan) and local diagnostic laboratories in Bathinda (Punjab) and Hyderabad (Andhra Pradesh). Patients were informed by the doctor about the test prior to collection of samples and the test for culture and sensitivity was conducted (based on prescription and doctor’s advice). UTI was confirmed by positive urine culture reports. All patients who had significant bacteriuria (>105 cfu/ml) were included for further microbiological analysis in the present study. Only one specimen per patient was included.
Sample collection and processing
Discrete colonies obtained after culturing the urine sample on Luria’s Broth (LB) agar plates were selected and these isolates were used to grow new colonies on the same media to ensure purity of isolated bacterial strains. Bacterial inocula were then prepared by suspending the freshly-grown bacterial colonies in 10 mL sterile LB and incubated at 37oC; which were then inoculated in both Hichrome UTI agar and MacConkey agar plates followed by incubation at 37oC for 24-48 h for bacterial identification based on specific metabolism of chromogenic substrates. Susceptibility of the isolated UTI causing bacteria to commonly used antimicrobial agents was then examined.
Antibiotic sensitivity testing
All antibiotic discs (Ampicillin 10μg; Gentamicin 30μg; Cefuroxime 30μg; Amikacin 30μg; Ciprofloxacin 5μg) and media used were obtained from Himedia Labs; India. The isolates were tested for antimicrobial susceptibility testing by the standard Kirby-Bauer disc diffusion method [11]. LB agar plates were incubated for 24h after inoculation with organisms and placement of discs. After 24h the inhibition zones were measured. Results were interpreted based on the diameter of the observed zone of inhibition. Following the Clinical and Laboratory Standards Institute Guidelines; the obtained results were categorized into three groups namely Sensitive (S); Intermediate (I); Resistant (R) and results were interpreted accordingly [12].
Statistical Analysis
The data were analyzed using Chi-square (χ2) test, confidence interval (CI), odds ratio (OR) analysis and p-value using SPSS 16 software. Statistical significance was defined when p-value was <0.05.
Results
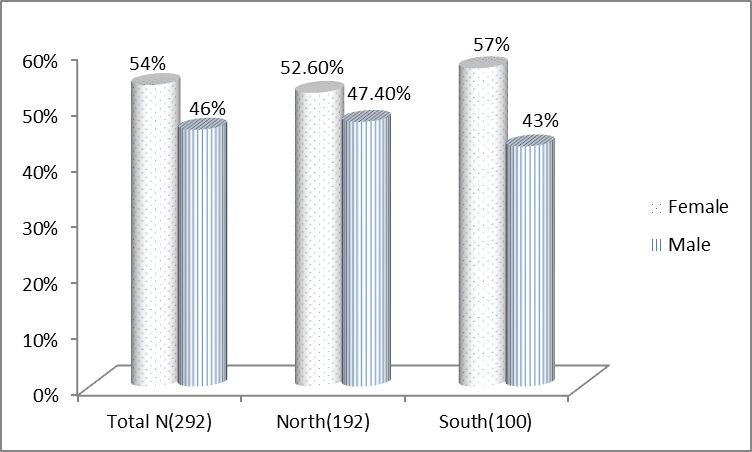
A total of 830 urine samples from clinically suspected patients were analysed for CA-UTI. Of these, 292 (35.1%) samples (192 from northern India and 100 from Southern India) were found to be culture positive showing significant bacteriuria and the remaining 538 (64.9%) samples showed either non-significant bacteriuria or were sterile. The incidence of the bacteria implicated in UTI in women was found higher than men [Table/Fig-1]. The total incidence of infection in women and men was 54% and 46% respectively, same pattern was observed in both the geographical regions.
Gender distribution of UTI Incidence during the study period.
[Table/Fig-2] illustrates the overall frequency of community-acquired uropathogens. From total 292 significant isolates, E.coli was the most pre-dominant isolate causing CA-UTI (55.1%), followed by E. faecalis, K. pneumoniae, Staphylococcus aureus (S. aureus), Pseudomonas aeruginosa (P. aeruginosa) and Proteus mirabilis (P. mirabilis) in order. Our study shows clear variation in prevalence of causative agents with geographical locations as seen from the [Table/Fig-2]. K. pneumoniae is second most common uropathogen after E. coli in the states from northern India while no K. pneumoniae was seen in samples from southern Indian population. Similarly P. mirabilis infection was also seen only in samples from northern Indian patients.
Distribution of microbiological flora causing urinary tract infections in OPD patients (Percentages given in parentheses)
| S.No | Organism | Frequency Total (N=292) | Frequency North (N=192) | Frequency South (N=100) |
|---|
| 1 | E. coli | 161 (55.1) | 107 (55.7) | 54 (54) |
| 2 | E. faecalis | 46 (15.8) | 17 (8.9) | 29 (29) |
| 3 | K. pneumoniae | 40 (13.7) | 40 (21) | 0 (0) |
| 4 | S. aureus | 18 (6.2) | 13 (6.8) | 5 (5) |
| 5 | P. aeruginosa | 13 (4.5) | 3 (1.6) | 10 (10) |
| 6 | P. mirabilis | 12 (4.1) | 12 (6.3) | 0 (0) |
| 7 | Others | 2 (0.7) | 0 (0) | 2 (2) |
Overall AMR profiles of the bacterial isolates are summarized in [Table/Fig-3]. All the clinical isolates showed highest resistance to ampicillin and least resistance towards amikacin (97.6% and 14% respectively).
Overall antimicrobial resistance profiles of the bacterial isolates (AMK: Amikacin; GEN: Gentamicin; CIP: Ciprofloxacin; CXM: Cefuroxime; A: Ampicillin)
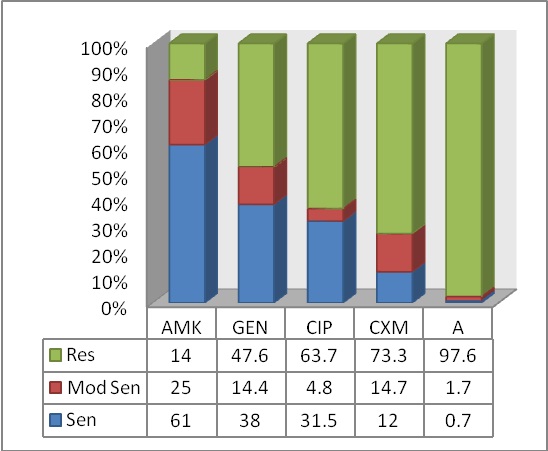
Among all the isolates, E. coli and P. mirabilis showed highest resistance to most commonly used antimicrobials except amikacin. Importantly for E. coli, the commonly recommended antimicrobials i.e. ampicillin, cefuroxime, ciprofloxacin and gentamicin showed high resistance rates (98.1, 84.5, 80.7 and 63.2%, respectively). The presence of P. aeruginosa, only 4.5% of all isolates was striking since it is considered to be a nosocomial pathogen. It showed highest sensitivity to gentamicin, ciprofloxacin and amikacin [Table/Fig-4].
Susceptibility profile of clinical isolates to commonly used antibiotics.
| Antibiotic | Gentamicin | Ampicilin | Amikacin | Cefuroxime | Ciprofloxacin |
|---|
| Organism | S | MS | R | S | MS | R | S | MS | R | S | MS | R | S | MS | R |
|---|
| E.coli (161) | 45 (28) | 14 (8.7) | 102 (63.4) | 02 (1.2) | 01 (0.6) | 158 (98.1) | 128 (79.5) | 13 (8.1) | 19 (11.8) | 16 (9.9) | 09 (5.6) | 136 (84.5) | 25 (15.5) | 06 3.7) | 130 (80.7) |
| E. faecalis (46) | 29 (63) | 09 (19.6) | 08 (17.4) | 00 (0.0) | 01 (2.2) | 45 (97.8) | 13 (28.3) | 23 (50) | 10 (21.7) | 05 (10.9) | 13 (28.3) | 28 (60.9) | 26 (56.5) | 01 (2.2) | 19 (41.3) |
| K. pneumoniae (40) | 17 (42.5) | 18 (45) | 05 (12.5) | 0 ( 0.0) | 02 (5.0) | 38 (95.0) | 06 (15.0) | 33 (82.5) | 01 (2.5) | 08 (20.0) | 18 (45.0) | 14 (35.0) | 16 (40) | 04 (10) | 20 (50) |
| S. aureus (18) | 07 (38.9) | 00 (0.0) | 11 (61.1) | 00 (0.0) | 01 (5.6) | 17 (94.4) | 10 (55.6) | 02 (11.1) | 06 (33.3) | 02 (11.1) | 01 (5.6) | 15 (83.3) | 10 (55.6) | 01 (5.6) | 07 (38.9) |
| P. aeruginosa (13) | 10 (76.9) | 01 (7.7) | 02 (15.4) | 00 (0.0) | 01 (0.0) | 13 (100) | 09 (69.2) | 02 (15.4) | 02 (15.4) | 04 (30.8) | 01 (7.7) | 08 (31.5) | 10 (76.9) | 00 (0.0) | 03 (23.1) |
| P.mirabilis (12) | 01 (8.3) | 00 (0.0) | 11 (91.7) | 00 (0.0) | 00 (0.0) | 12 (100) | 11 (91.7) | 00 (0.0) | 01 8.3) | 00 (0.0) | 00 (0.0) | 12 (100) | 5 (41.7) | 00 (0.0) | 07 (58.3) |
Statistical analysis showed significant variation in efficacy of using gentamicin, amikacin and ciprofloxcacin in southern and northern India while ampicillin and cefuroxime showed the same effect in both the geographical regions. The bacterial isolates from southern Indian patients as compared to north Indian patients were found to be 6 times and 2.5 times more susceptible to gentamicin and ciprofloxacin respectively. While north Indian isolates were about 3.5 times more susceptible to amikacin than south Indian isolates [Table/Fig-5].
Table showing the variation in antimicrobial resistance pattern of clinical isolates
| Total N=292 | Resistant cases | Chi Square (p value) | OR (95% CI)* |
|---|
| South N=100 (%) | North N=192 (%) |
|---|
| Gentamicin | 21 (21%) | 118(61.4%) | <0.001 | 5.99 (3.41,10.52) |
| Ampicillin | 98 (98%) | 187 (97.3%) | 0.749 | 0.763 (0.14, 4.00) |
| Amikacin | 24 (24%) | 17 (8.85%) | <0.001 | 0.308 (0.15, 0.60) |
| Cefuroxime | 69 (69%) | 145 (75.5%) | 0.178 | 1.44 (0.844, 2.48) |
| Ciprofloxacin | 50(50%) | 136 (70.8%) | <0.001 | 2.43 (1.47, 4.00) |
*Comparison of antibiotic resistance of uropathogens in South vs North India.Resistance % out of total in parentheses
Discussion
This study provides valuable laboratory data to monitor the status of AMR among uropathogens and to improve treatment recommendations in a specific geographical region. Our data were restricted to patients who can afford laboratory analysis; therefore this study may not reflect the true prevalence of UTI among patients in a particular geographical area. From total 830 urine samples collected from CA-UTI patients 292 (35.1%) yielded significant pathogens. A similar value of 39.7% was obtained by Oladeinde et al. in rural community from Nigeria [13]. The culture positive rate for CA-UTI was higher in our study in comparison with studies reported from Aligarh, India (10.86%) [5]. Another study has reported even higher incidence of uropathogens, 49% [14]. In the present cohort E. coli was the commonest uropathogen responsible for CA-UTI followed by E. faecalis and K. pneumoniae. The proportion of bacterial species isolated was similar to those described in previous studies [15–18]. The data collected from other places around the world, also shows that E. coli and K. pneumoniae are still the commonest uropathogens isolated in CA-UTI patients [19–21]. Our study showed statistically strong correlation between efficacy of an antibiotic and variation in geographical region. Hence, monitoring of antibiotic susceptibility of bacterial isolates in the community should be made mandatory for disease surveillance programs in a given area.
Our data shows that the most common isolate E. coli has become highly resistant to several commonly used antibiotics ampicillin, ciprofloxacin, ciprofloxacin and gentamicin. These high resistant rates among uropathogenic isolates from a particular community points to the selection pressures that generate, maintain and spread resistant strains in the community. It is also a fact that inappropriate clinical practices; mismanagement; unsupervised use; overuse; over the counter availability; lack of awareness and self-medication have worsened the condition in developing counties like India. Unqualified practitioners, untrained pharmacists and nurses all over the country use antimicrobials indiscriminately [22]. Similar practices have also been reported from other developing countries, such as Nepal and Vietnam [23–25]. Our findings strongly suggest that empirical treatment with these drugs should no longer be practiced.
Conclusions
The worldwide trend of empirically treating CA-UTI may worsen the debacle of growing AMR and certainly does not apply for specific geographical regions, where decreased susceptibility rates are documented for common uropathogens. Therefore, development of regional surveillance programs is necessary for implementation of CA-UTI guidelines.
*Comparison of antibiotic resistance of uropathogens in South vs North India.Resistance % out of total in parentheses
[1]. Gonzalez CM, Schaeffer AJ, Treatment of urinary tract infection: What’s old, what’s new, and what worksWorld J Urol 1999 17:372-82. [Google Scholar]
[2]. Arjunan M, Al-Salamah AA, Amuthan M, Prevalence and antibiotics susceptibility of uropathogens in patients from a rural environment, TamilnaduAm J Inf dis 2010 6:29-33. [Google Scholar]
[3]. Zelikovic I, Adelman RD, Nancarrow PA, Urinary tract infections in children. An updateWest J Med 1992 157:554-61. [Google Scholar]
[4]. Ahmed SM, Avasara AK, Urinary tract infections (UTI) among adolescent girls in Karimnagar District, AP K.A.P STUDYIndian J Pre Soc Med 2008 39:12-15. [Google Scholar]
[5]. Akram M, Shahid M, Khan AU, Etiology and antibiotic resistance patterns of community acquired urinary tract infections in JNMC Hospital, Aligarh, IndiaAnn Clin Microbiol Antimicrob 2007 6:6-11. [Google Scholar]
[6]. Omoregie R, Erebor JO, Ahonkhai I, Isobor JO, Ogefere HO, Observed changes in the prevalence of uropathogens in Benin City, NigeriaNZJ Med Lab Sci 2008 62:29-31. [Google Scholar]
[7]. Omoregie R, Eghafona NO, Urinary tract infection among asymptomatic HIV patients in Benin City, NigeriaBr J Biomed Sci 2009 66:190-93. [Google Scholar]
[8]. Tambekar DH, Dhanorkar DV, Gulhane SR, Khandelwal VK, Dudhane MN, Antimicrobial susceptibility of some urinary tract pathogens to commonly used antibioticsAfr J Biotechnol 2006 5:1562-65. [Google Scholar]
[9]. Biswas D, Gupta P, Prasad R, Sinha V, Arya M, Kumar A, Choice of antibiotic for empirical therapy of acute cystitis in setting of high antimicrobial resistanceIndian J Med Sci 2006 60:53-58. [Google Scholar]
[10]. Kothari A, Sagar V, Antibiotic resistance in pathogens causing community acquired urinary tract infections in India: A multicentric studyJ Infect. Dev Ctries 2008 2:354-8. [Google Scholar]
[11]. Bauer AW, Kirby WM, Sherris JC, Turck M, Antibiotic susceptibility testing by a standardized single disk methodAm J Clin Pathol 1966 45:493-96. [Google Scholar]
[12]. Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing: Nineteenth Informational Supplement. CLSI document M100-S19. Wayne; PA: USA. Clinical and Laboratory Standards Institute, 2009 [Google Scholar]
[13]. Oladeinde BH, Omoregie R, Olley M, Anunibe JA, Urinary tract infection in a rural community of NigeriaN Am J Med Sci 2011 3:75-77. [Google Scholar]
[14]. Orrett FA, Urinary tract infection in general practice in a rural community in south TrinidadSaudi Med J 2001 22:537-40. [Google Scholar]
[15]. García-Morúa A, Hernández-Torres A, Salazar-de-Hoyos JL, Jaime-Dávila R, Gómez-Guerra LS, Community-acquired urinary tract infection etiology and antibiotic resistance in a Mexican population groupRev Mex Urol 2009 69:45-48. [Google Scholar]
[16]. Sood S, Gupta R, Antibiotic resistance pattern of community acquired uropathogens at a tertiary care hospital in Jaipur, RajasthanIndian J Community Med 2012 37:39-44. [Google Scholar]
[17]. Dias Neto JA, Martins AC, Tiraboschi RB, Domingos AL, Cologna AJ, Paschoalin Community acquired urinary tract infection: Etiology and bacterial susceptibilityActa Cir Bras 2003 18:33-36. [Google Scholar]
[18]. Khameneh ZR, Afshar AT, Antimicrobial susceptibility pattern of urinary tract pathogensSaudi J Kidney Dis Transpl 2009 20:251-53. [Google Scholar]
[19]. Selvakumar BN, Jasmine R, Antibiotic Susceptibility of ESBL-producing urinary isolates at a tertiary care hospital in Tiruchirapalli, South IndiaJ Med Sci 2007 7:443-46. [Google Scholar]
[20]. Bahadin J, Teo SS, Mathew S, Aetiology of community-acquired urinary tract infection and antimicrobial susceptibility patterns of uropathogens isolatedSingapore Med J 2011 52:415-20. [Google Scholar]
[21]. Manjunath GN, Prakash R, Annam V, Shetty K, Changing trends in the spectrum of antimicrobial drug resistance pattern of uropathogens isolated from hospitals and community patients with urinary tract infections in Tumkur and BangaloreInt J Biol Med Res 2011 2:504-07. [Google Scholar]
[22]. Bano K, Khan J, Begum RH, Munir S, Akbar N, Ansari JA, Patterns of antibiotic sensitivity of bacterial pathogens among urinary tract infections (UTI) patients in a Pakistani populationAfr J Microbiol Res 2012 6:414-20. [Google Scholar]
[23]. Rao GG, Risk factors for the spread of antibiotic-resistant bacteriaDrugs 1998 55:323-30. [Google Scholar]
[24]. Wachter DA, Joshi MP, Rimal B, Antibiotic dispensing by drug retailers in Kathmandu, NepalTrop Med Int Health 1999 4:782-88. [Google Scholar]
[25]. Larsson M, Kronvall G, Chuc NT, Karlsson I, Lager F, Hanh HD, Antibiotic medication and bacterial resistance to antibiotics: A survey of children in a Vietnamese communityTrop Med Int Health 2000 5:711-21. [Google Scholar]