Can Metabotropic Glutamate Receptor 7 (mGluR 7) be a Novel Target for Analgesia?
Shivaprakash G1, Punya Suvarna2, Sanjay Hadigal3, Priyanka Kamath4, Natesh Prabhu5, Ashok Shenoy K6, Pallavi LC7
1 Associate Professor, Department of Pharmacology, Kasturba Medical College, Manipal University, Manipal, Karnataka, India.
2 Undergraduate Medical Student, Department of Pharmacology, Kasturba Medical College, Manipal University, Mangalore, Karnataka, India.
3 Postgraduate Student, Department of Pharmacology, Kasturba Medical College, Manipal University, Mangalore, Karnataka, India.
4 Postgraduate Student, Department of Pharmacology, Kasturba Medical College, Manipal University, Mangalore, Karnataka, India.
5 Postgraduate Student, Department of Pharmacology, Kasturba Medical College, Manipal University, Mangalore, Karnataka, India.
6 Professor and Head, Department of Pharmacology, Kasturba Medical College, Manipal University, Mangalore, Karnataka, India.
7 Assistant Professor, Department of Physiology, Kasturba Medical College, Manipal University, Manipal, Karnataka, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Shivaprakash G, Associate Professor, Department of Pharmacology, Kasturba Medical College, Manipal University , LHH Road, Manipal-575001, Karnataka, india. Phone : +919449553742, E-mail : sivag1977@gmail.com
Introduction: The present study was carried out to study the role of metabotropic glutamate receptor 7 (mGluR7) using its agonist, N,N’-bis(diphenylmethyl)-1,-ethanediamine (AMN082) for nociceptive stimuli, in animal models. By conducting this research, we aim to introduce a novel target for acute pain management.
Objective: To study the role of metabotropic glutamate receptor 7 (mGluR7), in analgesia, using mGluR7 agonist AMN082 in animal models.
Materials and Methods: Swiss albino mice of either sex, weighing 20-30gm were used for the study. The animals were divided into 3 groups with 6 mice in each group: Control or Normal group received 0.5% methylcellulose in normal saline; Standard group received the drug tramadol HCl at 40mg/kg; and test group received drug AMN 082 at 5mg/kg. All the drugs were administered by intraperitoneal route. Hot plate test and Tail flick test were done to evaluate the analgesic effect of the drug. Reaction time for the end points in both the models were noted before drug administration at 0 min and after drug administration at 15, 30,60,90 and 120 min. Statistical analysis was done using One-Way-ANOVA followed by Tukeys post hoc test. p-value was considered significant at ≤ 0.05.
Results: The group that received AMN082 showed significantly lesser reaction time compared to normal and standard groups in both the analgesia models.
Conclusion: The mGluR 7 stimulation by an agonist AMN082, did not show analgesic effect but induced hyperalgesia in response to thermal nociceptive stimuli.
Analgesia, Hot plate test, Metabotropic, Tail flick test
Introduction
Pain management is one of the most important aspect of many diseases. Existing analgesic drugs can relieve pain, but are associated with cardiovascular, renal and gastrointestinal system adverse effects. So as to avoid these, novel drugs and targets need to be explored.
Metabotropic glutamate receptors belong to the G protein-coupled receptors (GPCRs) superfamily and their eight receptor subtypes (mGlu1–8 receptors) are classified into three major groups. Most of the group III mGlu receptors (mGlu 4, -6, -7, and -8 receptors) are located within the presynaptic active zone where they act as auto receptors mediating feedback inhibition of glutamate release [1–3]. In addition to mediating release inhibition, mGlu7 receptor also activates signalling pathways that potentiate release. Release potentiation requires at least a 10 min exposure to a receptor agonist [4]. Once the mechanism of release potentiating is established, it occludes the release inhibition produced by a second addition of the agonist [4]. This complex intracellular pathway makes it interesting to study how agonist drugs behave in acute and chronic settings.
N,N’-bis(diphenylmethyl)-1,-ethanediamine (AMN082) is the allos–teric agonist of mGluR7. Earlier studies with this drug have reported: antidepressant–like effects; anxiolytic-like effects; inhibition of inflammatory pain-induced and incision-induced hypersensitivity; attenuation of allodynia and hyperalgesia and inhibition of cocaine-induced reinstatement of drug-seeking behaviour [5–9].
There are few studies that demonstrated the action of AMN082 on nociception with regard to acute and chronic analgesia [7]. Therefore, the present study was carried out to study the role of metabotropic glutamate receptor 7(mGluR7) agonist, AMN082 in acute analgesia, in animal models. By conducting this research, we aim to introduce mGluR7 as a novel target for acute pain management in many diseases like rheumatoid arthritis, etc. This could be a novel safer target as its mechanism is different from the currently available analgesics which causes serious adverse effects.
Objective
To study the role of metabotropic glutamate receptor 7 (mGluR7), in analgesia, using mGluR7 agonist AMN082 in animal models.
Materials and Methods
This study was conducted in the Department of Pharmacology, KMC Mangalore, India, from 1/oct/2013 to 25/oct/2013 after obtaining the approval from institutional animal ethics committee and in accordance with the CPCSEA guidelines. The study is intended to evaluate the role of mGluR 7 after inducing acute thermal nociceptive stimuli by administering agonist AMN082 in Swiss albino mice.
Animals
Swiss albino mice of either sex (procured from the Institutional Animal House) weighing 20-30gm were used for the study. The animals were kept under standard laboratory conditions, housed in animal room with alternate light dark cycle of 12 h each, at constant temperature of 25±1oC, with food and water, ad libitum. The animals were acclimatized to the laboratory conditions for 7 days, prior to the experiment.
Drugs and treatments
AMN082 (ABCAM, Allied Scientific Products, 8A, Aftab Mosque Lane, Kolkata) is a selective metabotropic glutamate receptor 7 (mGluR7) allosteric agonist. The drug was dispersed in a suspension of 0.5% methylcellulose (Sigma-Aldrich), which was used as a vehicle and is administered by intraperitoneal (IP) route at the dose of 5mg/kg. Dose of AMN082 was chosen based on previously published studies [7].
The control group received methylcellulose dissolved in normal saline by IP route. Standard drug tramadol HCl (Zydus Healthcare, East Sikkim, India) was administered by IP route at a dose of 40mg/kg. Test drug was administered at 5mg/kg by IP route. To test the analgesic effects, the drug was administered and observed for end points at 0 min (predose) and at 15, 30, 60, 90 and 120 min after administration.
Study groups
The animals were divided into 3 groups with 6 mice in each group.
Control or Normal: 0.5% methylcellulose in normal saline
Standard drug tramadol HCl: 40mg/kg
Test: AMN082: 5mg/kg
This test consists of introducing a mouse into an open-ended cylindrical space with a floor consisting of a metallic plate that is heated by a thermode. A plate heated to a constant temperature produces two behavioural components that can be measured in terms of their reaction times, namely paw licking and jumping. Paw licking and jumping were considered to be supraspinally integrated responses.
In this model, animals having basal reaction time not exceeding 15 sec were included in the study. Animals were placed individually on Eddy’s hot plate maintained at 55±10C and the reaction was noted either by licking the paw or jumping or raising the limb; whichever was observed first, was taken as the end point. The mean of two pre-drug recordings are taken as basal value (0 min). Observations were made after drug administration at 15, 30, 60, 90 and 120 min. A cut off time of 15 sec was maintained to prevent tissue injury.
The tail flicking is spinally integrated response. The tail flick test is performed using an analgesiometer. The animals were exposed to noxious stimuli (radiant heat) and tail flick latencies (the time taken to flick the tail, i.e., the reaction time) were noted. The mean of two pre-drug recordings were taken as basal value (0 min). The reaction time for each group was measured before and after drug administration at 15, 30, 60, 90 and 120 min. A cut off time of 15 sec was observed, to prevent tissue injury.
Statistical Analysis
Statistical analysis was done using One-Way-ANOVA followed by Tukeys post hoc test. SPSS Version 17 was used for statistical analysis. p-value was considered significant at ≤ 0.05.
Results
Hot plate test
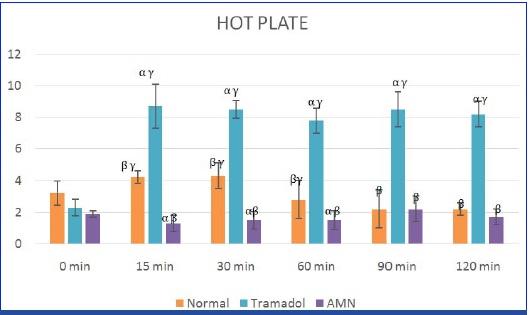
In this study the analgesic effect of AMN082 was evaluated by hot plate test. The group that received AMN082 showed lesser reaction time compared to standard group. This was statistically significant at 15min, 30min, 60min, 90min and 120min [Table/Fig-1]. The test group also showed lesser reaction time at 15, 30 and 60 min when compared to normal group.
Evaluation of analgesic effect of AMN082 drug by hot plate method.
X axis: Time in minutes at different intervals; Y axis: Time in seconds for the response; α β γ represent P value ≤0.05 compared to groups normal, tramadol and AMN respectively; AMN: N, N’-bis(diphenylmethyl)-1,-ethanediamine (AMN082). Normal group received 0.5% methylcellulose in normal saline; Standard group received tramadol at 40mg/kg and the test group received AMN at 5mg/kg
Tail flick test
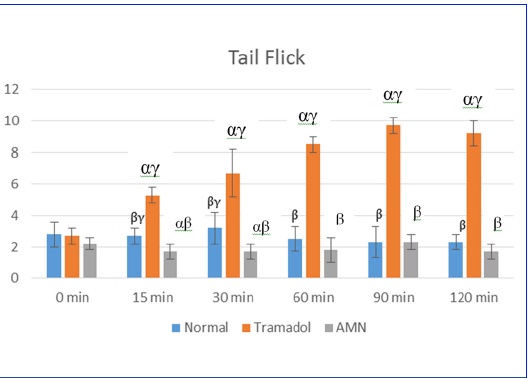
The tail flick latency was lesser in the test group compared to standard group. This was statistically significant at 15min, 30min, 60min, 90min and 120min following the drug administration [Table/Fig-2]. The test group also showed lesser reaction time when compared to normal group at 15 and 30 min.
Evaluation of analgesic effect of AMN082 by tail flick method.
X axis: Time in minutes at different intervals; Y axis: Time in seconds for the response; α β γ represent P value ≤0.05 compared to groups normal, tramadol and AMN respectively; AMN: N, N’-bis(diphenylmethyl)-1,-ethanediamine (AMN082). Normal group received 0.5% methylcellulose in normal saline; Standard group received tramadol at 40mg/kg and the test group received AMN at 5mg/kg
Discussion
The hot plate and tail flick tests are the most commonly used animal models to test for analgesic potential of an agent. The methods deal on the principle of thermal radiation heat [12]. The tail flick test is predominantly a response at spinal level and hot plate test response is at supraspinal level. These methods are mainly used for the evaluation of centrally acting analgesics.
There are no conclusive reports on the role of the mGluR 7 in pain. AMN082 is an orally active allosteric modulator with full agonist activity at mGluR7 and has the ability to cross blood brain barrier [13]. There were handful of studies which showed a variable effect of AMN082 on nociception.
A pronociceptive role for mGluR7 is supported by findings that intra-amygdala infusion of AMN082 decreases spinal withdrawal reflex thresholds and increases vocalization of normal rats following mechanical compression of the knee joint [14]. Similarly, periaqueductal infusions of AMN082 was also found to be pronociceptive [15]. However, Michael C et al., concludes that activation of mGluR7 may be pro- or anti-nociceptive, depending on its primary site of action [16].
In our study AMN082 did not show analgesic effect in tail flick and hot plate test. Instead, the test group when compared to normal group showed lesser reaction time at 15 and 30 min in tail flick test and at 15, 30 and 60 min in hotplate test implying hyperalgesia. The reason for hyperalgesia is not known. Most of the group III glutamate receptors are autoreceptors believed to modulate glutamate [1–3]. Further studies on exploring the possibility of modulation of other mediators release and their sensitization is required for understanding its exact mechanism. The reason for hyperalgesia diminishing after 30 and 60 min respectively in tail flick test and hot plate test may indicate the recovery from hyperalgesia following decreasing drug concentration.
On the contrary, other studies have demonstrated the antinociceptive effects of AMN082 in attenuating thermal hyperalgesia, following intrathecal and intraperitoneal administration [7,8]. The reason for the above discrepancy is not known. Dolan S et al., demonstrated the analgesic response to thermal stimuli in rat model following post carrageenan and post-surgical incision model [7]. Osikowicz M et al., in neuropathic mouse pain model concludes that the analgesic effect was observed only with Von Frey test at one single dose of 3 mg/kg given intraperitoneally (IP) [8]. Von Frey test detects animal sensitivity to mechanical stimuli. The reason for efficacy observed only to mechanical stimuli but not to thermal stimuli needs more information by future studies with different tests which can detect animal sensitivity to different nociceptive stimuli and with different doses.
Conclusion
We studied the role of mGluR7 receptors in analgesia using its agonist, AMN082 in mice. The mGluR 7 stimulation by an agonist AMN082 did not show analgesic effect but induced hyperalgesia in response to thermal stimuli.
[1]. Shigemoto R, Kulik A, Roberts JD, Target-cell-specific concentration of a metabotropic glutamate receptor in the presynaptic active zoneNature 1996 381(6582):523-25. [Google Scholar]
[2]. Forsythe ID, Clements JD, Presynaptic glutamate receptors depress excitatory monosynaptic transmission between mouse hippocampal neuronesJ Physiol 1990 429:1-16. [Google Scholar]
[3]. Herrero I, Vazquez E, Miras-Portugal MT, Sanchez-Prieto J, Decrease in [Ca2+] but not in cAMP Mediates L-AP4 inhibition of glutamate release: PKC-mediated suppression of this inhibitory pathwayEur J Neurosci 1996 8(4):700-09. [Google Scholar]
[4]. Martín R, Durroux T, Ciruela F, Torres M, Pin JP, The metabotropic glutamate receptor mGlu7 activates phospholipase C, translocates munc-13-1 protein, and potentiates glutamate release at cerebrocortical nerve terminalsJ Biol Chem 2010 285(23):17907-17. [Google Scholar]
[5]. Bradley SR, Uslaner JM, Flick RB, Lee A, Groover KM, The mGluR7 allosteric agonist AMN082 produces antidepressant-like effects by modulating glutamatergic signalingPharmacol Biochem Behav 2012 101(1):35-40. [Google Scholar]
[6]. Stachowicz K, Branski P, Klak K, van der Putten H, Cryan JF, Flor PJ, Selective activation of metabotropic G-protein-coupled glutamate7 receptor elicits anxiolytic-like effects in mice by modulating GABAergic neurotransmissionBehav Pharmacol 2008 19(5-6):597-603. [Google Scholar]
[7]. Dolan S, Gunn MD, Biddlestone L, Nolan AM, The selective metabotropic glutamate receptor 7 allosteric agonist AMN082 inhibits inflammatory pain-induced and incision-induced hypersensitivity in ratBehav Pharmacol 2009 20(7):596-604. [Google Scholar]
[8]. Osikowicz M, Mika J, Makuch W, Przewlocka B, Glutamate receptor ligands attenuate allodynia and hyperalgesia and potentiate morphine effects in a mouse model of neuropathic painPain 2008 139(1):117-26. [Google Scholar]
[9]. Li X, Li J, Gardner EL, Xi ZX, Activation of mGluR7s inhibits cocaine-induced reinstatement of drug-seeking behavior by a nucleus accumbens glutamate-mGluR2/3 mechanism in ratsJ Neurochem 2010 114(5):1368-80. [Google Scholar]
[10]. Eddy NB, Leimback B, Synthetic analgesics:11 DithyienylbuttylaminesJ Pharmacol Exp Ther 1953 3:544-47. [Google Scholar]
[11]. Armour FE, Smith DL, A method for determining loss of pain sensationJ Pharmacol Exp Ther 1941 72:74-79. [Google Scholar]
[12]. Medhi B, Prakash A, Animal experiments on central nervous systemIn: Practical Manual of Experimental and Clinical Pharmacology 2010 Ist edNew DelhiJaypee Brothers Medical Publishers:201-02. [Google Scholar]
[13]. Conn PJ, Niswender CM, mGluR 7’s lucky numberProc Natl Acad Sci U S A 2006 103(2):251-52. [Google Scholar]
[14]. Palazzo E, Fu Y, Ji G, Maione S, Neugebauer V, Group III mGluR7 and mGluR8 in the amygdala differentially modulate nocifensive and affective pain behaviorsNeuropharmacology 2008 55(4):537-45.[PubMed: 18533199] [Google Scholar]
[15]. Marabese I, Rossi F, Palazzo E, de Novellis V, Starowicz K, Cristino L, Periaqueductal gray metabotropic glutamate receptor subtype 7 and 8 mediate opposite effects on amino acid release, rostral ventromedial medulla cell activities, and thermal nociceptionJournal of neurophysiology 2007 98(1):43-53.[PubMed: 17507496] [Google Scholar]
[16]. Montana Michael C, Gereau Robert W. IV, Metabotropic glutamate receptors as targets for analgesia: antagonism, activation, and allosteric modulationCurr Pharm Biotechnol 2011 12(10):1681-18. [Google Scholar]