Out of 741 diagnosed patients of scabies, 240 patients were included in this study, those attended OPDs of Department of Dermatology, Venereology and Leprology of Patna Medical College Hospital, Patna, from 1st April 2011 to 31st March 2012. This study protocol was approved by Institutional Ethics Committee of Patna Medical College, Patna. Written informed consent was taken from patients during their enrolment for study. The patient related data, medical history, diagnosis, laboratory values and given treatment was noted in a case record form.
Materials and Methods
Two hundred and forty patients were randomly allocated to four Groups. Each group contained 60 patients and treatment given was as follows.
Group 1: Ivermectin 200 g/kg body weight (IVER) Oral single dose
Group 2: Permethrin 5% cream (PM) Topical single application
Group 3: Gamma benzene hexacloride1% (GBHC) lotion Topical single application
Group 4: Benzyl Benzoate 25% (BB) lotion Topical single application
In Group 2, 3 and 4 drug was applied topically below the jaw line after scrub bath and left overnight. All of the patients were followed up for improvement at the end of 1st wk and 6th wk.
Parameters used to compare the efficacy of the Groups by seeing improvement in
Severity of pruritus.
Severity of the disease.
Severity of pruritus is evaluated by Visual Analogues scale (VAS). VAS was defined as a 10 cm line, in which point 0 (zero) refers to existence of no pruritus and point 10 refers to the most severe pruritus. According to this scale, we scored pruritus of the patients.
Point 1 to 3: Mild pruritus
Point 4 to 6: Moderate pruritus
Point 7 to 10: Severe pruritus
Severity of the disease is measured according to the number of lesions present. It can be graded as:
Mild: < 10 lesions.
Moderate : 11 – 49 lesions.
Severe : > 50 lesions.
A pre-structured proforma was used to collect the relevant information (patients data, clinical finding etc) at baseline, 1st wk and at 6th wk of follow-up or if any complaints in between.
Statistical analysis of the data was done by Chi-square test, degree of freedom and p-values.
Results
Shown in [Table/Fig-1,2,3,4,5]
Response to treatment in various groups
| Improvement seen with severity of pruritus as parameter |
|---|
| At 1st follow-up No.of cases (%) | At 2nd follow-up No.of cases (%) |
|---|
| Oral Ivermectin single dose 200μg/kg body wt. | 31 (51.66%) | 20 (33.34%) |
| Topical Permethrin 5% cream single application | 38 (63.34%) | 16 (26.66%) |
| Topical GBHC 1% lotion single application | 26 (43.33%) | 19 (31.66%) |
| Topical BB 25% lotion single application | 30 (50.00%) | 11 (18.33%) |
Assessments of severity of pruritus at 2nd follow up (end of 6th week)
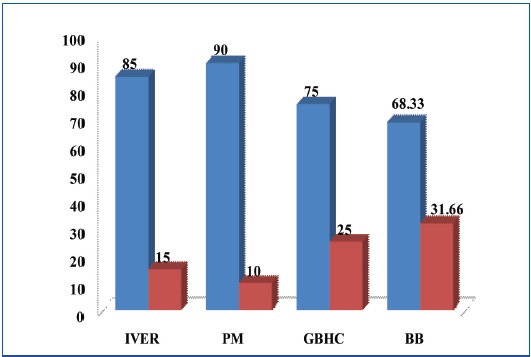
Improvement seen with severity of lesions as parameter
| Improvement seen with severity of lesions as parameter |
|---|
| At 1st follow-up No.of cases (%) | At 2nd follow-up No.of cases (%) |
|---|
| Oral Ivermectin single dose 200μg/kg body wt. | 32 (53.34%) | 16 (26.60%) |
| Topical Permethrin 5% cream single application | 43 (71.66%) | 10 (16.66%) |
| Topical GBHC 1% lotion single application | 28 (46.66%) | 15 (25.00%) |
| Topical BB 25% lotion single application | 29 (48.33%) | 10 (16.66%) |
Assessments of severity of lesions at 2nd follow up (end of 6th week)
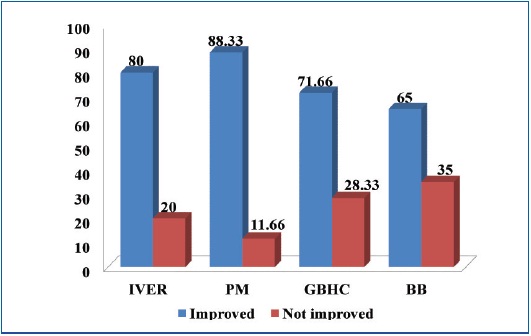
Comparison of results of various Groups at the end of 6 wks
| Group | No.of cases | Severity of pruritus at 6th week | Severity of lesion at 6th week |
|---|
| Improved No. (%) | Not improved No. (%) | Improved No. (%) | Not improved No. (%) |
|---|
| IVER | 60 | 51(85) | 9(15) | 48(80) | 12(20) |
| PM | 60 | 54(90) | 6(10) | 53(88.33) | 7(11.66) |
| GBHC | 60 | 45(75) | 15(25) | 43(71.66) | 17(28.33) |
| BB | 60 | 41(68.33) | 19(31.66) | 39(65) | 21(35) |
| Chi-Square Test | x2 =10.54 | p=0.01 p<.05,(S) | x2 =10.19 | p=0.02 p<.05,(S) |
| Degree of freedom (D.F.) | D.F.=3 | D.F.=3 |
| Difference Between Groups (p-Values) | 1-2 | x2 =0.80 | D. F.= 1 | p=0.37(NS) | x2 =1.57 | D. F.=1 | p=0.21(NS) |
| 1-3 | x2 =1.88 | D. F.= 1 | p=0.17(NS) | x2 =1.13 | D. F.=1 | p=0.29(NS) |
| 1-4 | x2 =4.66 | D. F.= 1 | p=0.03(S) | x2 =4.38 | D. F.=1 | p=0.04(S) |
| 2-3 | x2 =4.68 | D. F.= 1 | p=0.03(S) | x2 =5.21 | D. F.=1 | p=0.02(S) |
| 2-4 | x2 =8.02 | D. F.= 1 | p=0.005(S) | x2 =9.13 | D. F.=1 | p=0.002(S) |
| 3-4 | x2 =0.66 | D. F.= 1 | p=0.42(NS) | x2 =0.62 | D. F.=1 | p=0.43(NS) |
x2 =Chi-Square Test, p<0.05=Significant(S), D.F.=Degree of freedom, p>0.05= Not Significant (NS)
Discussion
Response to treatment in various Groups
Oral Ivermectin given single dose (200 μg/kg body weight): In our study at the end of 2nd follow up complete improvement was seen in 85% and 80% when severity of pruritus and severity of lesion were taken as parameters respectively. Usha and Gopala [4] found that a single dose of Ivermectin provided a cure rate of 70%, which increased to 95% with 2 doses given at 2 wk interval. The lower efficacy of single dose Ivermectin could reflect the lack of ovicidal action of the drug. Thus, the results of the present study are comparable with other studies which have a cure rate of >80% [5–7].
Topical Permethrin 5% cream single application: Earliar studies [4,8–10] have reported cure rate >80% with Permethrin. Higher cure rates (98%) was reported after two applications. In our study cure rate with single application has been studied. At 1st follow up 63.34% showed cure rate which increased to 90% at 2nd follow up.
Topical GBHC 1% lotion: Earlier studies [8,11–13] have reported <75% cure rate with 1% topical GBHC lotion. Nag et al.,[13] have reported a cure rate of 68% only with 2% GBHC 2 applications in a day. In our study single application of Topical GBHC 1% lotion has shown a cure rate of 75% at 2nd follow up.
Topical application of B.B. lotion 25%: Our study has been done with BB lotion 25% single application. At 2nd follow up 68.33% reported improvement in pruritus and 64.99% reported cure in severity of lesion. Earlier studies [12,14] by different researchers have been done with 10-20% of BB lotion. Sampaio [15] showed 57% of patients improved after treating with benzyl benzoate. Brooks and Grace [16] found improvement in 51% patients at the end of 3 wks.
Comparison of statistical significance among four Groups
[Table/Fig-5] shows comparison of results among four Groups at the end of 6 wks considering severity of pruritus and severity of the disease as a parameter of efficacy. Difference in efficacy of Group 1 (Ivermectin) therapy was statistically non-significant with Group 2 (Permethrin) and 3 (Gamabenzene hexachloride), but statistically significant with Group 4 (Benzyl bezoate). This shows that efficacy of Group 1 therapy was comparable to Group 2 and Group 3 therapy but more efficacious than Group 4 therapy.
Group 2 therapy was more efficacious than Group 3 and 4 because difference in efficacy was statistically significant. Difference in efficacy of Group 3 and 4 therapies was statistically non-significant.
Thus the finding of present study has statistical validation between groups.
Although topical agents carried certain drawbacks, our study reported that single application of Permethrin 5% gave maximum response when severity of pruritus and severity of lesion were taken as parameters to compare efficacy of different Groups thus making it the most effective treatment and therefore suitable to be the treatment of choice.
Response to single oral dose of Ivermectin 200 μg/kg body wt. was slightly low when compared to Topical Permethrin but higher when compared to response obtained with either topical GBHC or topical Benzyl Benzoate. Though the results obtained with single oral dose of Ivermectin (200 μg/kg body wt) (i.e. patients belonging to Group 1 were slightly low compared to Permethrin, patient acceptance was very good especially among students living in hostels, where inadequate facilities for bathing and taking a good scrub bath were the major hurdle in topical application.
Despite the need for further exploration, oral ivermectin could be a viable alternative for management of scabies especially where compliance to topical scabicides is improbable or impractical.
Higher cost and lower efficacy of single dose oral Ivermectin as compared to topical Permethrin supports consideration of initial therapy with Permethrin wherever possible. However, Oral Ivermectin can be used where topical scabicides fail.
Further study with combined oral and topical agents, repeated administration and use of softening agents to treat hyperkeratosis and increase efficacy of topical scabicides needs to be done for this benign but transmissible condition.
A significant reduction in disease burden is possible only when along with appropriate scabicides, treatment of all contacts and clothings are done simultaneously and the underlying environmental and social conditions that promote infectious skin diseases are addressed.
Improvement in literacy and economic status along with awareness about personal hygiene can definitely bring down the prevalence of this disease.
Conclusion
The results suggested that oral Ivermectin and topical Permethrin (5%) were equally efficacious and therefore suitable to be the treatment of choice. Oral Ivermectin is well tolerated, non irritant to skin, does not show central nervous system side effects because it does not cross blood brain barrier. So the good therapeutic response with few side effects seen with oral Ivermectin can be useful in those patients for whom topical treatment is potentially irritant and less well tolerated. Oral Ivermectin could be a valuable drug in mass community treatment in managing epidemics, in treating complicated cases, where topical scabicides fail, where Permethrin resistance is encountered or where non compliance is a problem with topical agents.
x2 =Chi-Square Test, p<0.05=Significant(S), D.F.=Degree of freedom, p>0.05= Not Significant (NS)