Acebrophylline, which contains ambroxol and theophylline-7 acetic acid, has been found to improve ciliary clearance, reduce the frequency of episodes of bronchial obstruction, reduce the need for β2-agonists and improve indices of ventilatory function. The anti-inflammatory effect of acebrophylline may be useful in the treatment of this disease. Unlike other xanthine derivatives including theophylline, it has minimal side effects like palpitations and tachycardia [6].
Asthma causes considerable impairment in the physical, social and emotional aspects of lives of patients and thus has a substantial impact on the quality of life [7]. These outcomes are important because they reflect the patients’ burden of disease rather than a simple assessment of clinical and economic perspectives.Most of the studies comparing the combinations of anti-asthmatic drugs with inhaled corticosteroids have been carried out in the United States and Europe. Such studies are scarce from the Indian subcontinent. So this study was undertaken with the following objectives: a) To compare the efficacy of three different classes of anti – asthmatic drugs i.e., long acting β2 – agonist (formoterol), leukotriene antagonist (montelukast) and acebrophylline when used in combination with budesonide in patients with bronchial asthma in a tertiary care hospital and to assess the safety of these drugs b) To determine their role in the improvement of quality of life of patients.
Materials and Methods
This observational, prospective, comparative study was done on patients of bronchial asthma, after getting the approval of the Institutional Ethics Committee (IEC) in a tertiary care hospital in coastal Karnataka in South India. The study was carried out for a period of six months during 2012-2013. Patients of bronchial asthma on inhalational steroid therapy who needed an add-on therapy and who were willing to give written informed consent were included in the study. Those who were already on the study drugs, those with present or past history of smoking and those with old or active pulmonary tuberculosis, COPD, bronchiectasis or any other respiratory diseases or cardiovascular diseases were excluded from the study.
After taking informed consent, the demographic details of the patients were recorded. The details of inhalational steroid medication taken, routine blood test reports and spirometry values were noted. The quality of life was assessed using ‘Asthma Quality of Life Questionnaire’ (AQLQ). A total of 75 patients were included in the study. Patients received the following medications: Patients in first group received formoterol (6 mcg/puff) + budesonide (100 mcg/puff) combination inhaler (2 puffs twice daily), while those in second group received montelukast (10 mg once daily) + budesonide (100 mcg/puff, 2 puffs twice daily) and those in third group received acebrophylline (100 mg twice daily) + budesonide (100 mcg/puff, 2 puffs twice daily). Patients were followed-up for a total of 4 wk period after the initiation of treatment. After 2 wk of treatment, quality of life was assessed again and occurrence of any adverse event was documented. After 4 wk of treatment, the routine blood test reports, repeat spirometry values, quality of life and adverse events were recorded.
The Asthma Quality of Life Questionnaire (AQLQ) was used to assess the improvement in the quality of life [8]. There were 29 questions in the AQLQ which were divided into 4 domains. Domain A dealt with activity limitation while domain B dealt with occurrence of symptoms like shortness of breath, wheezing, cough, heaviness of chest and night time asthmatic episodes. Domain C dealt with emotional disturbances due to asthma like frustration or concern due to the disease, concern about availability and intake of medicines and also the fear of episodic attacks. Domain D dealt with precipitation of asthmatic attacks on exposure to environmental stimuli like cigarette smoke, dust, air pollution, strong smells or perfumes. Patients were asked to think about how they had been during the previous 2 wk and then respond to the questions. They were asked to grade the responses to each of the 29 questions on a four-point scale (0= no impairment/ none of the time, 1= mild impairment/ some of the time, 2= moderate impairment/ most of the time, 3= severe/persistent impairment/ all the time). The mean score of all the questions in each domain was calculated and this was taken as the individual domain scores. The overall AQLQ score was calculated as the mean of the individual domain scores. The questionnaire was administered to the patients at baseline visit, after 2 wk and 4 wk of treatment.
Statistical Analysis
The data collected was analysed using SPSS software version 16.0. Categorical variables were analysed using repeated measures ANOVA, followed by Bonferroni’s post-hoc test. A p-value < 0.05 was considered significant.
Results
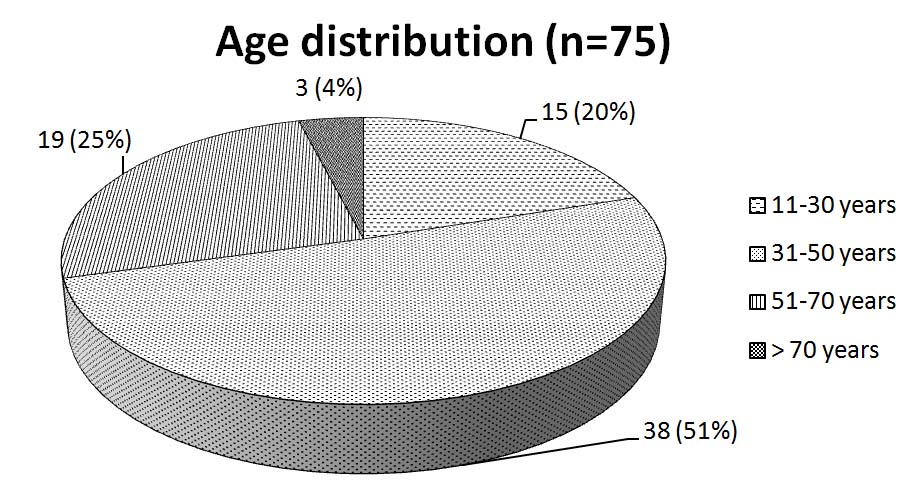
A total of 75 patients who were diagnosed with asthma and put on the study treatments were followed up for a period of 1 month after treatment initiation. Of these, 36 were males and 39 were females. The male to female ratio was 0.92:1. This indicates a female preponderance to asthma. The study revealed peak incidence in the age group of 31-50 y [Table/Fig- 1] The baseline laboratory investigations in all patients were within normal limits. There was no significant difference in their values when repeated after four wk of treatment in all the three study groups.
Age distribution of study population
Lung Function Tests
The forced expiratory volume in one second (FEV1) and Peak Expiratory Flow Rate (PEFR) showed significant improvement after 4 wk of treatment [Table/Fig- 2,3] .But on intergroup comparison, no significant difference was observed. Gastric irritation was observed only in acebrophylline-treated group in 3 patients (12%) which occurred usually about half an hour after the intake of the drug. It was mild in severity and lasted for about an hour. It was relieved spontaneously and did not warrant discontinuation of the study drug.
Pre and post treatment values of FEV1 (L)
| Groups (n)* | At Baseline visit | After 4 wk of treatment | p-value |
|---|
| Group 1 (25) | 2.12 ± 0.65 | 2.38 ± 0.68 | <0.01 |
| Group 2 (25) | 2.24 ± 0.75 | 2.47 ± 0.73 | <0.01 |
| Group 3 (25) | 2.27 ± 0.70 | 2.47 ± 0.78 | <0.01 |
*n = number of patients in each group. Values expressed as mean ± SD, p< 0.05 being considered significant
Pre and post treatment values of PEFR (L/sec)
| Groups (n) † | At Baseline visit | After 4 wk of treatment | p-value |
|---|
| Group 1 (25) | 4.72 ± 1.30 | 5.16 ± 1.35 | <0.01 |
| Group 2 (25) | 5.80 ± 1.92 | 6.21 ± 1.82 | <0.01 |
| Group 3 (25) | 5.56 ± 1.84 | 6.10 ± 1.90 | <0.01 |
., †n = number of patients in each group. Values expressed as mean ± SD, p< 0.05 being considered significant
Quality of Life Questionnaire scores
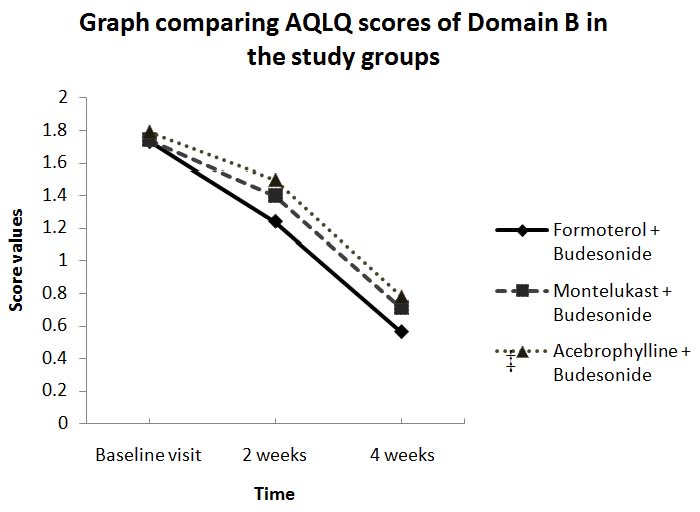
There was a significant decrease in the mean scores of all the four domains and also the overall AQLQ score after 4 wk of treatment in all the groups. Comparative analysis of the three groups revealed no significant difference in domains A, C and D and the overall AQLQ score. However, in domain B, the formoterol group showed a significantly better control of symptoms when compared with montelukastandace brophylline groups [Table/Fig- 4].
Graph comparing AQLQ scores of Domain B in the study groups., Values expressed as mean ± SD., p< 0.05 being considered significant. (p = 0.012) ‡
Discussion
In this study the number of female patients was more than males. Other studies done in India and Europe have reported higher incidence of asthma in females [9–11]. This has been attributed to a greater expression of symptoms of the disease in females, higher female life expectancy, hormonal factors and the fact that women are normally more exposed than men to cleaning products and other irritants. The mean age of the patients was 43.75 ± 14.31 y with peak incidence in the age group of 31-50 y. A lower mean age of patients [10] and a peak incidence above 60 y of age are revealed in certain studies [11]. This variation in asthma incidence in different age groups could be due to the small sample size of our study. Asthma is associated with airway eosinophilia which can be correlated with the severity of the disease [4]. In this study, there was no significant difference seen in the blood eosinophil counts before and after treatment in all the three study groups. Significant reduction in blood eosinophil counts were seen with montelukast but not with LABA in some studies [12]. However, few studies report no significant change in mean eosinophil counts after 6 wk of montelukast treatment [13].
FEV1 represents the air flow in both small and large airways. A decreased FEV1 is associated with an increased risk of severe exacerbations of asthma. Regular monitoring of pulmonary function is particularly important for asthma patients who do not perceive their symptoms until airflow obstruction is severe. The significant improvement seen in the average FEV1 in all the treatment groups in this study is consistent with other studies as well [1,2,14]. Comparative analysis between the study groups showed no significant difference in the average FEV1 which is consistent with other studies [12]. However, authors also found that LABA showed better efficacy in improving FEV1 than montelukast [15,16].
PEFR measures the airflow through the bronchi and thus the degree of obstruction in the airways. In clinical trials, peak flow values have been used as major outcome measures to monitor short and long term asthma control and treatment responses,. The significant improvement seen in the average PEFR in all the treatment groups is consistent with other studies [1,16]. On intergroup comparison, there was no significant difference in the change in PEFR which is consistent with other literature reports [17]. However, in some studies, LABA showed better efficacy in improving PEFR than montelukast [15,16].
In our study, 3 patients (12%) on acebrophylline treatment reported mild gastric irritation whereas no adverse effects were reported in formoterol and montelukast groups. Adverse events like headache (0.8%), tremors (1.6%) and tachycardia (0.65%) have been reported with the use of formoterol [2]. Authors have also reported headache (1% each) with the use of both LABA and montelukast, and insomnia (1%) with the use of LABA [16]. Another study reported a lesser incidence of gastrointestinal upset (2.6%) with acebrophylline [18].
The significant decrease seen in the mean scores of all domains and the overall AQLQ score after 4 wk of treatment in all the treatment groups of this study is in accordance with other studies [19]. The superiority in symptom control with formoterol can be attributed to its longer duration of action when compared with montelukast or acebrophylline; thereby leading to lesser occurrence of asthmatic symptoms.
The results of the study show that acebrophylline can provide a third option as an add-on therapy to ICS. The combination of acebrophylline with ICS represents a logical option as this combination offers different mechanisms of action for relief of asthma and also the additional anti-inflammatory effect of acebrophylline can benefit in the treatment of asthma. The follow-up period was restricted to one month which is ideally not sufficient to assess the effects of the drugs on the pulmonary functions. This was done because acebrophylline is usually administered for a brief period and not many safety reports are available on the long term usage of acebrophylline in asthma treatment. Another limitation was that the exacerbations in symptoms within the study period and the usage of rescue medication (short acting β2–agonist like salbutamol) could not be elicited accurately. Hence studies with larger sample size and of longer duration of treatment are warranted to establish the long term benefits and risks of different therapeutic combinations in the treatment of asthma.
Conclusion
Formoterol, montelukast or acebrophylline, when combined with inhaled corticosteroids have shown similar efficacy in the treatment of asthma. Also, formoterol and montelukast were better tolerated than acebrophylline. All the drugs have shown similar effects on the quality of life of patients but formoterol appeared to have better symptom control.