Teeth like bone are composed of both organic and inorganic components. Bulk of the tooth is made up of dentin forming the main part of crown. Central core within the dentin is formed by soft tissue component called pulp which is a specialized loose connective tissue containing fibers, cells, blood vessels, nerve terminations and ground substance [1].
Inorganic component of teeth can be studied in ground sections, but decalcification is required to study the organic components [2]. Moreover, the pulpal soft tissue which otherwise is difficult to appreciate in the ground sections, can be easily assessed in decalcified sections [3]. Decalcification is routinely used technique in most histopathological laboratories for the microscopic examination of calcified tissues [4]. The purpose of decalcification is to remove calcium salts from mineralized tissue, resulting in preservation of organic components [5]. Various methods have been employed for decalcification including use of heat, vacuum,electric current and chemical agents [6]. Amongst them, the chemical agents are the most commonly used for routine histopathological analysis. The most widely used chemical agents for decalcification are either acids, which react with calcium in bone or teeth to form soluble calcium salts or chelating agents which form a complex with calcium. The effect of these agents depends on various factors, like their concentration used, temperature and time taken for decalcification [7].
The use of these chemicals may lead to some alterations in the tissue architecture including staining characteristics [8]. As the pulp contains the soft tissue components, it is the most affected tissue during decalcification [9]. The best decalcifying agent would be the one that allows complete removal of calcium with minimal damage to cells and tissue, works rapidly and provides adequate staining characteristics [7]. So, the aim of this study was to perform a qualitative analysis of tissue preservation and to compare the efficacy of various decalcifying agents on human permanent teeth including both hard and soft tissue components and thus determining the most efficient decalcifying agent for the diagnostic purposes.
Materials and Methods
The study was conducted in the department of Oral & Maxillofacial Pathology at Maharishi Markandeshwar College of Dental Sciences & Research, Mullana, Ambala, India. Freshly extracted, non carious, non attrited, 60 human permanent teeth including, incisor, canine, premolar and molar were obtained from the patients aged 40-45 y. Within 2-4 h of extraction, the access opening was done for each tooth, using a high speed carbide bur with air rotor and apical 1/3rd of root was cut, for better penetration of fixative agent and decalcifying fluid. 10 % formalin was injected, without pressure, inside the root canal to fix the pulpal tissue. The teeth were fixed in the formalin for one day. After that, the specimens were exposed to different decalcifying solutions. The five decalcifying agents used in the study were 10 % Nitric acid (HNO3),10% Formal nitric acid (FNA), 10 % Formic acid (FA), 8% Potassium formate (KF) + 8% Formic acid (FA), Neutral Ethylenediamenetetracetic acid (EDTA).
The study was divided into three sets: set I, set II and set III. Each set comprised of 5 decalcifying solutions having 4 teeth i.e. one incisor, one canine, one premolar and one molar in each solution. Decalcification was carried out at room temperature by suspending the teeth in the container with the help of a thread in such a way that the teeth were completely immersed in about 100 ml of the solution. Time at the start of decalcification was noted. The solutions were subjected to repeated agitation and replaced by freshly prepared solutions every 24 h. The end point of decalcification was measured by physical method followed by chemical and radiographic methods. The physical method involved bending, needling and probing at the cervical area of tooth using a fine needle or probe every day. The chemical method employed was calcium – oxalate test, which involved the detection of calcium by precipitation of insoluble Ca (OH)2 or calcium oxalate in decalcifying solution. This method was performed for all the acidic solutions but for EDTA, the test was done by acidifying the solution [1]. Chemical test was performed every time the solution was changed.When completion of decalcification was confirmed by physical and chemical tests, radiographs were taken daily for all the solutions until complete radiolucency was seen [Table/Fig-1].The radiographic method was performed by placing the tooth 15 cm in front of X-Ray source and exposed for 6 sec. After confirming the decalcification by all the three methods, the teeth were removed from solutions and washed under running tap water for 24 h.
Radiographs of one of set of samples taken during the study: changes from radiopacity to radiolucency depicted the completion of decalcification procedure

In the set I it was observed that the specimens were not soft enough for the section cutting, although the end point of decalcification was achieved. So in the next set i.e. set II, the specimens were kept for two more days and it was found that there was no difficulty in section cutting. The specimens of set II were subjected to routine tissue processing, sectioning and staining with hematoxylin and eosin. The stained sections were observed under light microscope by three independent observers and graded from 0-2 (0 - Total loss of tissue architecture, 1 – Partially preserved tissue architecture, 2 – Well preserved tissue architecture) separately for hard and soft tissues based on the following criteria [Table/Fig-2,3,4,5,6]:
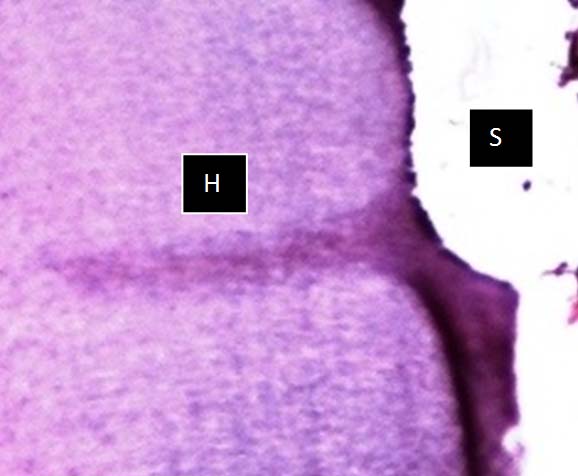
Photomicrograph of HNO3 solution, showing well preserved hard (H) & soft (S) tissue architecture with very good staining
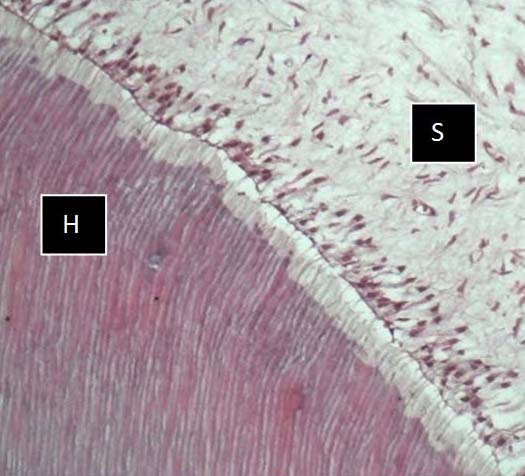
Photomicrograph of FNA solution, showing good preservation of hard (H) & soft (S) tissue with good staining, but less as compared to HNO3
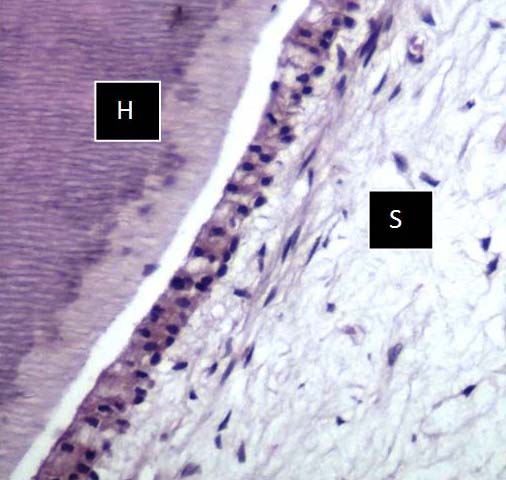
Photomicrograph of EDTA Solution showing preservation of hard (H) & soft (S) tissue with moderate staining, but less as compared to HNO3 &FNA solution
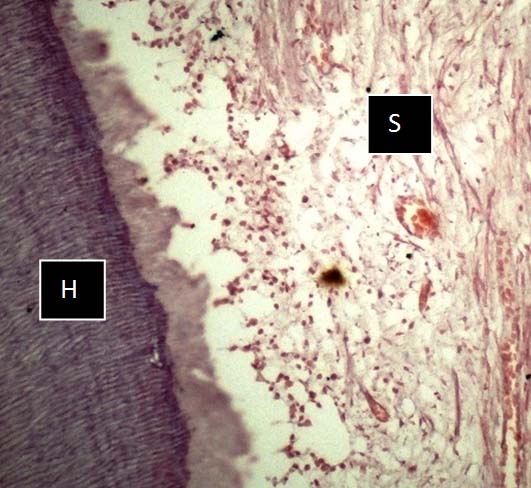
Photomicrograph of KF + FA solution showing limited hard (H) & soft (S) tissue preservation and compromised staining quality
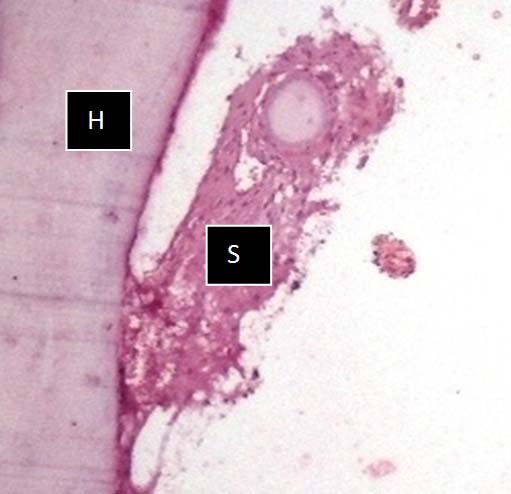
Photomicrograph of FA solution showing very poor hard (H) & Soft (S) tissue preservation and compromised staining quality
For hard tissue:
Preservation of dentinal structure
Clarity of dentinal tubules
Staining characteristics.
For soft tissue:
Pulpal organization
Preservation of ectomesenchyme
Preservation of odontoblastic layer
Staining characteristics.
The scores for all four types of teeth were added up to give a single score for each solution which theoretically ranged from 0-8. To evaluate the further effect of decalcifying agents, the time of decalcification was increased for each solution by another 2 days in set III. For this set, it was observed that there was shredding during section cutting.
Results
Different decalcifying agents were evaluated and compared on the basis of selected parameters. It was observed that 10% FNA decalcified the teeth fastest, followed by 10 % HNO3, 10 % FA, 8 % KF + 8% FA and EDTA being the slowest [Table/Fig-7].
Days required for decalcification
| Decalcifying solutions | Days required |
|---|
| 10% Formal nitric acid (FNA) | 3-5 days |
| 10% Nitric acid (HNO3) | 5-7 days |
| 10% Formic acid (FA) | 10-12 days |
| 8% Potassium formate (KF) + 8% Formic acid (KF+FA) | 18 -20 days |
| Ethylenediaminetetraacetic acid (EDTA) | 28 -30 days |
It was also observed that specimens of set II showed ease of section cutting with better preservation of tissue and well defined staining characteristics. Preservation of tissue architecture with uniformity of staining was graded by three independent observers. To evaluate interobserver variability between three observers, paired t –test was applied and it was found that p-values obtained were statistically insignificant (p>0.05) for both hard and soft tissues [Table/Fig-8].
Paired sample t- test for inter-observer variation
| Variables | Between | Mean | SD | SE | t-value | p-value |
|---|
| Hard tissue | Observer 1 and 2 | 1.000 | 1.000 | .447 | 2.236 | .089 |
| Observer 1 and 3 | .800 | 1.095 | .490 | 1.633 | .178 |
| Observer 2 and 3 | -.200 | .837 | .374 | -.535 | .621 |
| Soft tissue | Observer 1 and 2 | .600 | .894 | .400 | 1.500 | .208 |
| Observer 1 and 3 | .800 | .837 | .374 | 2.138 | .099 |
| Observer 2 and 3 | .200 | .447 | .200 | 1.000 | .374 |
SD: Standard deviation, SE: Standard error of mean
Further, readings were subjected to one way ANOVA test for statistical analysis. Mean values of grading done by three observers for different decalcifying agents suggested that scoring for hard tissue preservation was better shown by 10 % HNO3(7.33) followed by EDTA (6.67), 10% FNA (6.33), 8% KF + 8% FA (5.67) and 10% FA (5.00). Similarly scoring for pulpal tissue was highest for 10% HNO3(7.00) followed by 10% FNA (6.33), EDTA (5.67), 10% FA (4.67) and 8% KF + 8% FA (4.00) [Table/Fig-9].
Oneway ANOVA descriptive analysis
| Tissue | Solution | N | Mean | SD | SE |
|---|
| Hard tissue | HNO3 | 3 | 7.33 | .577 | .333 |
| FNA | 3 | 6.33 | .577 | .333 |
| FA | 3 | 5.00 | 1.000 | .577 |
| KF+ FA | 3 | 5.67 | 1.155 | .667 |
| EDTA | 3 | 6.67 | .577 | .333 |
| Total | 15 | 6.20 | 1.082 | .279 |
| Soft tissue | HNO3 | 3 | 7.00 | .000 | .000 |
| FNA | 3 | 6.33 | .577 | .333 |
| FA | 3 | 4.67 | .577 | .333 |
| KF +FA | 3 | 4.00 | .000 | .000 |
| EDTA | 3 | 5.67 | 1.155 | .667 |
| Total | 15 | 5.53 | 1.246 | .322 |
DS: Standard deviation, SE: Standard error of mean
When Post Hoc test was applied for multiple comparisons between decalcifying agents,it was observed that for hard tissue, 10% HNO3 was showing significant difference (p<0.05) only with 10% FA (0.036) [Table/Fig-10]. Regarding pulpal tissue,10% HNO3 was showing significant difference (p<0.05) with 10 % FA (0.008) and 8 % KF + 8% FA(0.001). 8% KF + 8% FA was also showing significant difference with 10% FNA (0.008) [Table/Fig-11].
Post Hoc test for multiple comparisons between agents for hard tissue
| Solution (I) | Solution (J) | Mean difference (I-J) | Standard error | p- value |
|---|
| HNO3 | FNA | 1.000 | .667 | .585 |
| FA | 2.333 | .667 | .036* |
| KF | 1.667 | .667 | .166 |
| EDTA | .667 | .667 | .850 |
| FNA | HNO3 | -1.000 | .667 | .585 |
| FA | 1.333 | .667 | .332 |
| KF | .667 | .667 | .850 |
| EDTA | -.333 | .667 | .986 |
| FA | HNO3 | -2.333 | .667 | .036* |
| FNA | -1.333 | .667 | .332 |
| KF | -.667 | .667 | .850 |
| EDTA | -1.667 | .667 | .166 |
| KF+FA | HNO3 | -1.667 | .667 | .166 |
| FNA | -.667 | .667 | .850 |
| FA | .667 | .667 | .850 |
| EDTA | -1.000 | .667 | .585 |
| EDTA | HNO3 | -.667 | .667 | .850 |
| FNA | .333 | .667 | .986 |
| FA | 1.667 | .667 | .166 |
| KF | 1.000 | .667 | .585 |
* = Statistically significant
Post Hoc test for multiple comparisons between agents for soft tissue
| Solution (I) | Solution (J) | Mean difference (I-J) | Standard error | p- value |
|---|
| HNO3 | FNA | .667 | .516 | .702 |
| FA | 2.333 | .516 | .008* |
| KF | 3.000 | .516 | .001* |
| EDTA | 1.333 | .516 | .148 |
| FNA | HNO3 | -.667 | .516 | .702 |
| FA | 1.667 | .516 | .055 |
| KF | 2.333 | .516 | .008* |
| EDTA | .667 | .516 | .702 |
| FA | HNO3 | -2.333 | .516 | .008* |
| FNA | -1.667 | .516 | .055 |
| KF | .667 | .516 | .702 |
| EDTA | -1.000 | .516 | .359 |
| KF+FA | HNO3 | -3.000 | .516 | .001* |
| FNA | -2.333 | .516 | .008* |
| FA | -.667 | .516 | .702 |
| EDTA | -1.667 | .516 | .055 |
| EDTA | HNO3 | -1.333 | .516 | .148 |
| FNA | -.667 | .516 | .702 |
| FA | 1.000 | .516 | .359 |
| KF | 1.667 | .516 | .055 |
* = Statistically significant
Discussion
Decalcification is the commonly employed technique in most of the histopathology laboratories for the microscopic examination of calcified tissues including teeth and bones [4]. Many studies have been done by the researchers to introduce new decalcifying agents and to modify the presently used agents in order to meet the criteria of the most efficient decalcifying agent which ensures complete removal of calcium without causing any damage to tissue architecture and provide adequate staining characteristics. In the present study an attempt has been made to compare the efficacy of 5 different decalcifying agents for both hard and soft tissues components of human permanent teeth.
In the present study, we have done comparison between three sets of specimens in all the decalcifying agents and it was found that even after reaching the decalcification point by all the three methods, the specimens of set I resulted in difficulty of section cutting. In the set II, the time of decalcification was increased by two days in each solution and it was observed that the teeth were soft enough for section cutting. Further the time of decalcification was increased by two days for set III and it was found that there was shredding during section cutting and poor staining characteristics which can be due to the over decalcification of teeth. Therefore, it is necessitate determining the end point of decalcification, for the ease of section cutting and better results of staining needed in histopathological analysis. Various methods have been used for this purpose. In our study, end point of decalcification was determined using three methods: physical, chemical and radiographic. Physical test was done using bending and probing but as it can create artifacts; so it was not considered as an accurate method. Due to the complexity of the procedure chemical method was not suggested as a routine test for diagnostic purposes. Amongst them radiographic method was the most reliable to check the end point which is also in accordance with Gayle Callis [1].
One important criteria of an efficient decalcifying agent is the reasonable speed of decalcification. In our study speed of decalcification was highest for 10% FNA followed by 10% HNO3, 10% FA, 8 % KF + 8% FA and EDTA being the slowest. Similar results were obtained in the study done by Singh S, Sarkar K [2] where FNA decalcified the teeth fastest among the chemical agents used. Zappa et al., [10], Mattuella LG et al., [9], Singh S, Sarkar K [2] and Sanjai et al., [3] also found in their studies that the speed of decalcification was slowest by using EDTA. Maurine William AB [11] in his study reported that the time taken for decalcification by 5% HNO3 was 7-9 days for a single tooth, but in our study the time taken was less i.e. 5-7 days, this may be due to the increased concentration of HNO3 used which was 10%. This finding was in accordance with Culling who stated that the rate of decalcification also depends on the concentration used for the particular acid and varies accordingly [7].
On comparing the efficacy of various decalcifying agents in terms of preservation and staining characteristics of both hard and soft tissues, superior results were obtained with 10% HNO3 followed by 10% FNA and EDTA which was according to the respective mean values obtained. But statistically 10 % HNO3 was showing significant difference only with 10% FA for the hard tissue, which also showed the least scores. This means 10 % FA should not be used for diagnostic and research purposes as it causes maximal damage to the tooth integrity. Similarly for the soft tissue, 10% HNO3 was showing significant difference with 10% FA and 8% KF + 8% FA, and FNA was showing significant difference with 8% KF + 8% FA. Therefore, it is recommended that for pulp preservation it is better to avoid 10% FA and 8% KF + 8% FA as decalcifying agents, to provide excellent and reproducible results. However, Goland P et al., [12] noticed in their study that following fixation in reactive halogen compounds, such as dichloro-s-triazene, procion dyes and Lissatan PR, 5% FA as decalcifying agent better preserve the enamel as compared to HNO3 and HCl but they did not mention about the preservation of pulp in their study.
When efficacy was compared between 10% HNO3 and 10% FNA, no statistically significant difference was found but tissue integrity and staining was better observed with HNO3 which has high score; the reason could be the rapid action of FNA which can produce some alteration to the architecture of both hard and soft tissue components of tooth.Therefore, it is suggested that HNO3 should be preferred over FNA. In the present study, EDTA was also showing almost similar results as 10% HNO3 with little alterations in the tissue architecture, the reason for these alterations could be the prolonged time required for the completion of decalcification. Similarly, when Mattuella LG et al., [9] compared the efficacy of EDTA with Ana moarse solution for processing of human primary teeth with inactive carious lesion, they found that EDTA solution caused alterations in the cellular and tissue architecture due to its long time required for decalcification. Therefore, it is preferred to use a decalcifying agent which balances both the time for decalcification and tissue integrity.
According to the study done by Zappa et al., [10], HNO3 and FA were showing worst results after decalcification, for both hard and soft tissue components of tooth as compared to EDTA and other agents used in their study. Also, it has been found in the studies done by Singh S, Sarkar K [2] and Sanjai K et al., [3] that overall results were best shown by EDTA, in contrast to our study where HNO3 was showing better tissue preservation and staining quality.
Conclusion
An efficient decalcifying agent should preserve the tissue architecture with a reasonable speed of decalcification for the rapid diagnosis. In our study HNO3 showed the most efficient result as it balances both tissue integrity and time factor suggesting that it can be used as a stable decalcifying agent for routine histopathological diagnosis. However, further studies are required on a large sample size with consideration of individual factors to evaluate the effect of these agents on dental hard and soft tissuesand tofind a suitable decalcifying agent which provides reproducible results.
SD: Standard deviation, SE: Standard error of mean
DS: Standard deviation, SE: Standard error of mean
* = Statistically significant
* = Statistically significant