Introduction
COPD has been defined by The Global Initiative for Obstructive Lung Disease [1] (GOLD) as a disease state characterized by airflow limitation that is not fully reversible. The chronic airflow limitation characteristic of COPD is caused by a mixture of small airway disease (obstructive bronchiolitis) and parenchymal destruction (emphysema), the relative contributions of which vary from person to person [2].
COPD is a leading cause of morbidity and mortality worldwide and a major public health problem. It is a social and economic burden in our country regarding cost of treatment, loss of man-days. GOLD estimates suggest that COPD will rise from the sixth to the third most common cause of death worldwide by 2020. Presently, COPD is the fourth leading cause of death and affects >16 million people in the United States [3].
Over the past several decades, the use of drug therapy in COPD has expanded, and provides an objective and generally optimistic picture that such treatment is effective. Bronchodilators and anti-inflammatory agents are used in COPD to reverse broncho-constriction and improve airflow limitation. The goals of drug therapy are not only to improve lung function, but also to improve quality of life, exercise capacity, and prevent exacerbations [4].
Theophylline (1, 3 Dimethyl Xanthine) has been used as a bronchodilator in the management of COPD for several decades. The plasma concentration of therapeutic range was established to be 10 to 20 mg/L because of narrow therapeutic index. Theophylline may have anti inflammatory effects on small airways, reduction of hyperinflation and thus a reduction in dyspnea. The proposed mechanisms of action of Theophylline are Phosphodiesterase inhibition (nonselective), Adenosine receptor antagonism, increased interleukin-10 release, stimulation of catecholamine (epinephrine) Sectionrelease, inhibition of mediators (prostaglandins, tumor necrosis factor), inhibition of intracellular calcium release, inhibition of nuclear factor-κB (↓ nuclear translocation), increased apoptosis, ↑ Histone deacetylase activity (↑ efficacy of corticosteroids) [5].
Acebrophylline (Ambroxol + Theophylline-7-Acetate), another Xanthine derivative, is used as a bronchodilator for the treatment of bronchial asthma and COPD in adults. Acebrophylline modifies mucus secretion by lowering viscosity of ‘gel’ phase, increasing ‘sol’ phase and increases mucociliary clearance by augmenting ciliary motility. Acebrophylline inhibits intracellular phosphodiesterase and facilitates bronchial muscles relaxation by increasing cAMP levels. It selectively inhibits phosphatidyl choline and phospholipase A, TNF-alpha and leukotrienes. Inhibition of such pro-inflammatory mediators causes significant reduction of the airway inflammation and obstruction in chronic stages [6].
There are only few studies available on comparison between Acebrophylline and Sustained Release Theophylline in patients of COPD. The present study was designed to compare the efficacy of the two drugs in terms of lung function improvement and symptomatic benefit as well as tolerability/side-effects to help the patients to lead a socially and economically productive life.
Materials and Methods
The present study was conducted in the Department of Respiratory Medicine and Department of Physiology of a tertiary care hospital at Kolkata for 1 year. It was an open randomized longitudinal study of 6 weeks duration.
The inclusion criteria were 1) Adult patients of both sexes above 40 years attending respiratory medicine OPD, 2) Symptoms like dyspnoea, cough, sputum etc, 3) Spirometric assessment showing both of the following – a) post bronchodilator FEV1/FVC < 70% , b) FEV1 < 80% but > 50% of predicted, thus defining moderate COPD according to GOLD guidelines 2010[1,4] clinically stable for 6 weeks. Tiotropium inhalation 18μgm per day, through metered dose inhaler was routinely co-administered, as the use of Methylxanthine agent alone is not recommended by GOLD for moderate COPD patients.
Exclusion criteria were 1) Patients unwilling to give consent, 2) Other cardio-respiratory disorders such as bronchial asthma, allergy, atopy, pulmonary TB, pulmonary fibrosis, lung malignancy, myocardial infarction, heart failure, uncontrolled hypertension, 3) Acute exacerbation, 4) Severe or very severe COPD (GOLD grade 3 and 4) and 5) Pregnant and lactating female patients.
Informed consent was collected prior to inclusion in the study and the study protocol was approved by the institutional ethics committee.
Initially, 60 patients were enrolled; out of which 8 did not give consent, 7 did not follow inclusion and exclusion criteria and 5 failed to report on subsequent visits.
Following the screening visit, eligible patients entered a 2-week wash-out period to ensure clinical stability (i.e. no exacerbations). significantInhaled rescue salbutamol was permitted at any time but ≥6 h before pulmonary function tests (PFT). All the long acting β2 agonist, oral steroids and methylxanthines were withheld during this wash-out period. Patients who successfully completed this phase have entered into the study phase of 6-week periods. Patients were then randomized at a ratio of 1:1, according to the table generated by random allocation software into two groups. Group-1 patients received Acebrophylline (100 mg twice daily) and Group-2 patients received Sustained Release (SR) Theophylline (300 mg once daily). During the study period all the patients of either group received 18 μgm of Tiotropium as once daily dosage through metered dose inhaler.
Efficacy assessment
a) Spirometry - All the patients were investigated with electronic spirometer (model: Recorders and Medicare system’s RMS Helios 702) in the department of Physiology. Pulmonary function test parameters included were forced expiratory volume in one second (FEV1), FEV1/FVC ratio, peak expiratory flow rate (PEFR), forced expiratory flow from 25 to 75 % (FEF25-75). The results were recorded in percent of predicted values. Spirometry was performed at visit-1 (day ‘0’), visit-2 (day ‘21’) and visit-3 (day ‘42’). The same equipment was used throughout study and the test was performed according to a Standard Operative Protocol (SOP).
b) Clinical improvement was assessed by shortness of breath (SOB) in mMRC scale (modified Medical Research Council), presence of wheeze, and frequency of use of reliever medications during treatment.
Tolerability assessment
Evaluated by presence or absence of epigastric tenderness, pain chest, nausea, palpitation, tremor, tachycardia, dizziness, headache, insomnia and sleep disorder which was stated by the patients or found by the investigator at each visit during the study period. Detailed clinical examination was performed at 0, 21 and 42 days and if needed in between also.
Finally, all the data were analyzed by using SPSS version-17 for Mean±SD, student independent t-test and paired t-test.
Results
Total 40 patients were studied and randomly placed in two groups. The demographic parameters like age, BMI and relevant PFT parameters (FEV1, FEV1/FVC, FEF 25-75 and PEFR) of group-1(Acebrophylline) and 2 (SR Theophylline) is presented in [Table/Fig-1].
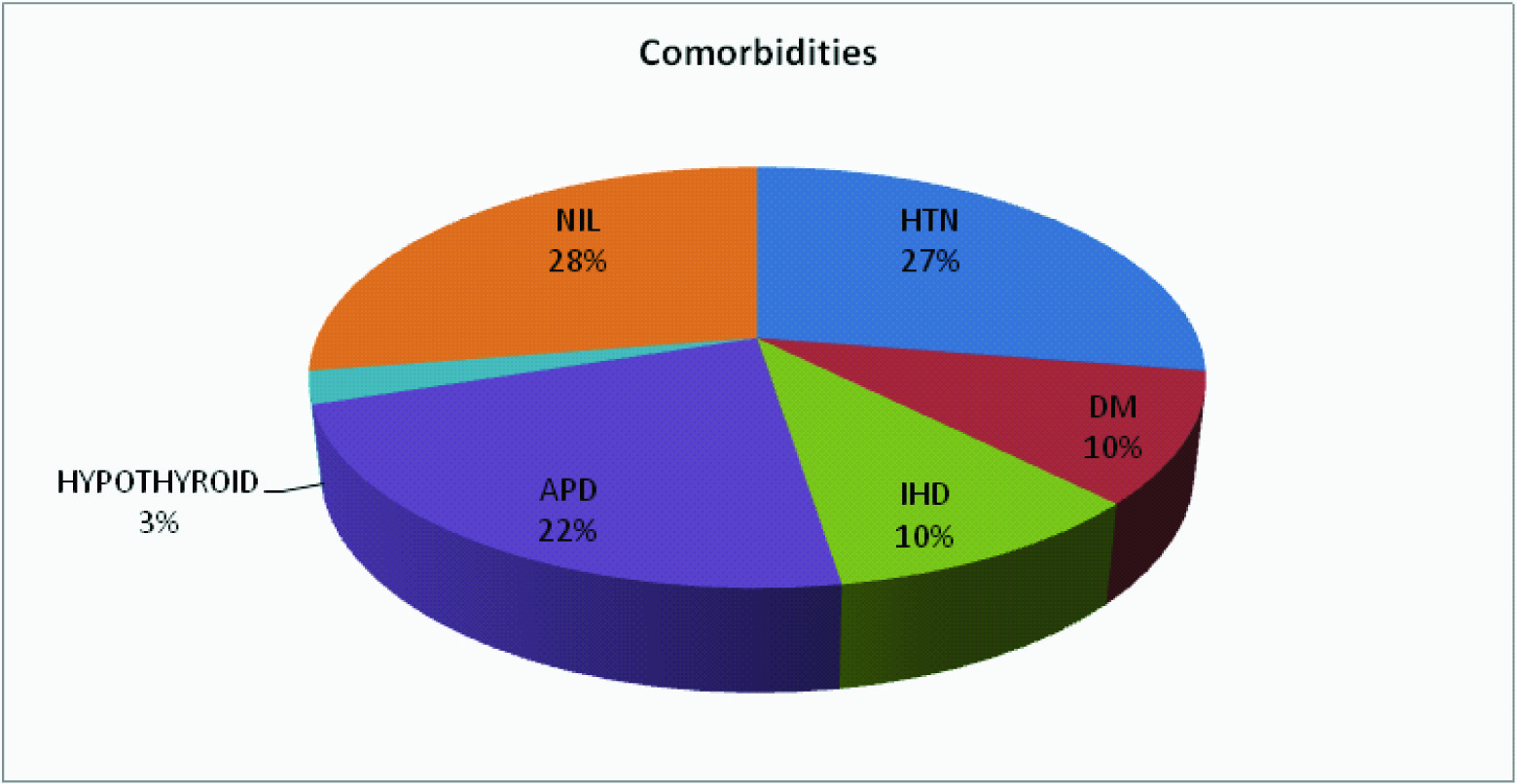
Among the 40 patients, 80% were male and 20% were female, 80% were smokers and 20% were non-smokers. [Table/Fig-2] shows the co-morbidities (27% hypertension, 10% ischemic heart disease (IHD), 22% acid peptic disorder (APD) and 10% were diabetic).
[Table/Fig-3] shows intra group comparison of the PFT parameters in visit-1, visit-2 and visit-3. In group-1, there was significant improvement of FEV1, FEV1/FVC and FEF 25-75 in all the three visits (p-value <0.05) whereas, there was significant change of PEFR in visit-3 compared to visit-1 (p-value=0.01). In patients of group-2 there was significant improvement of FEV1 and FEF25-75 in visit-2 and visit-3 compared to visit-1 (p-value <0.05) whereas in case of FEV1/FVC, there was significant change in visit-2 compared to visit-1 (p-value=0.0008). No significant change of PEFR was observed.
Inter group comparison of the PFT parameters between two groups is presented in [Table/Fig-4]. There was no significant change of the parameters in visit-2 and visit-3 (p-value>0.05).
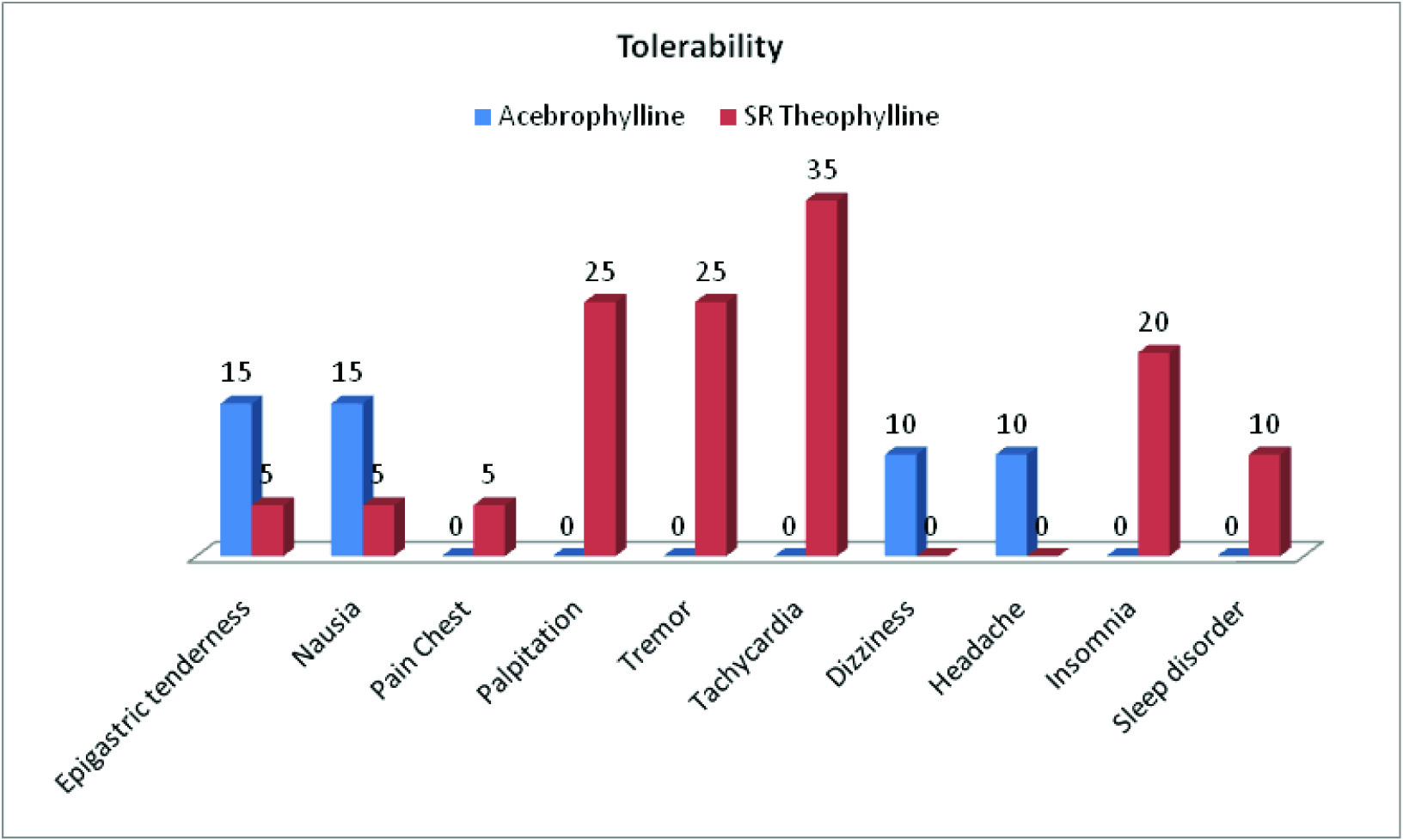
[Table/Fig-5] shows tolerability of the drugs used in the study. Pain chest, palpitation, tremor, tachycardia, insomnia and sleep disorder were absent with Acebrophylline whereas dizziness and headache were absent with SR Theophylline.
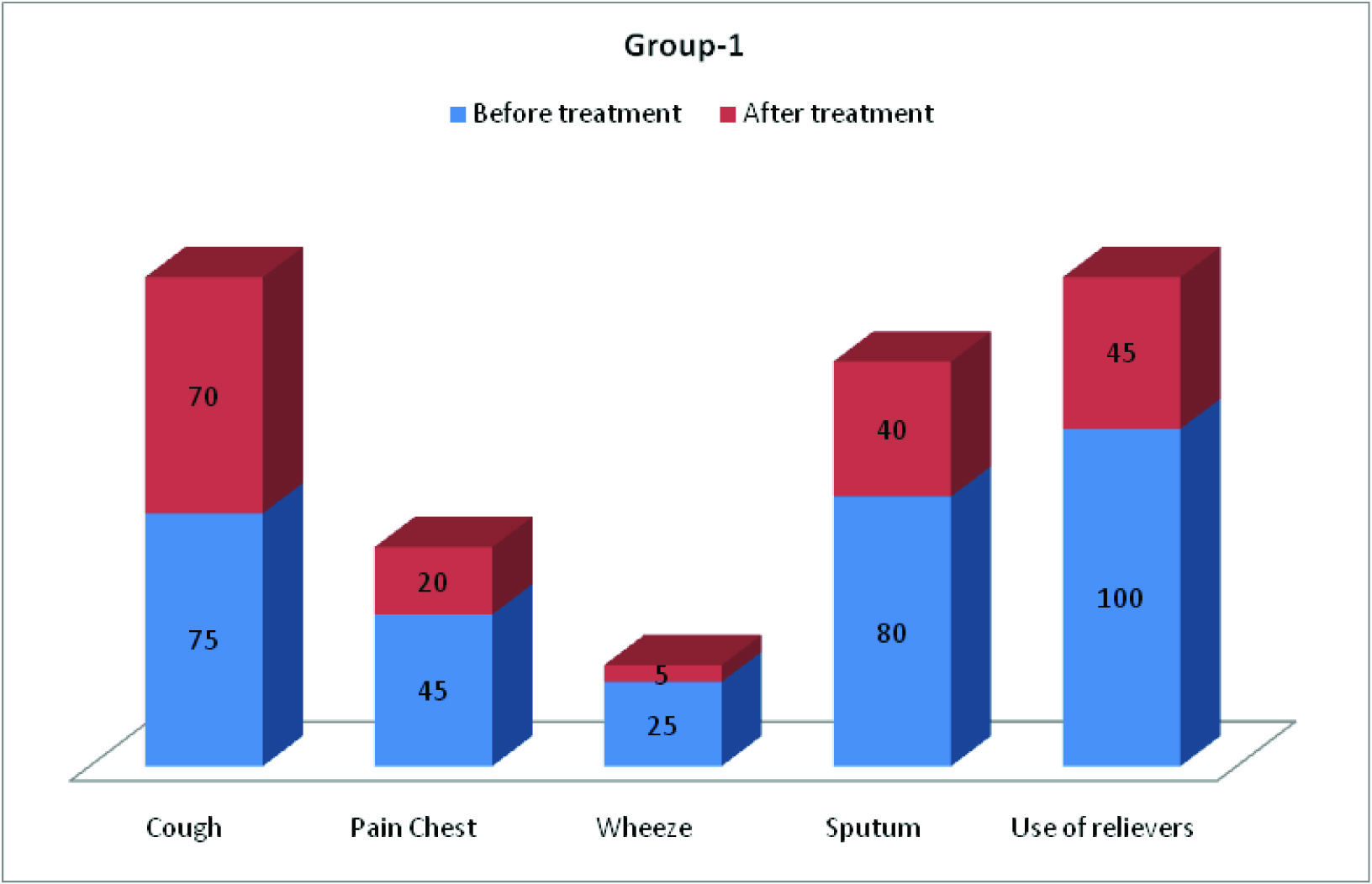
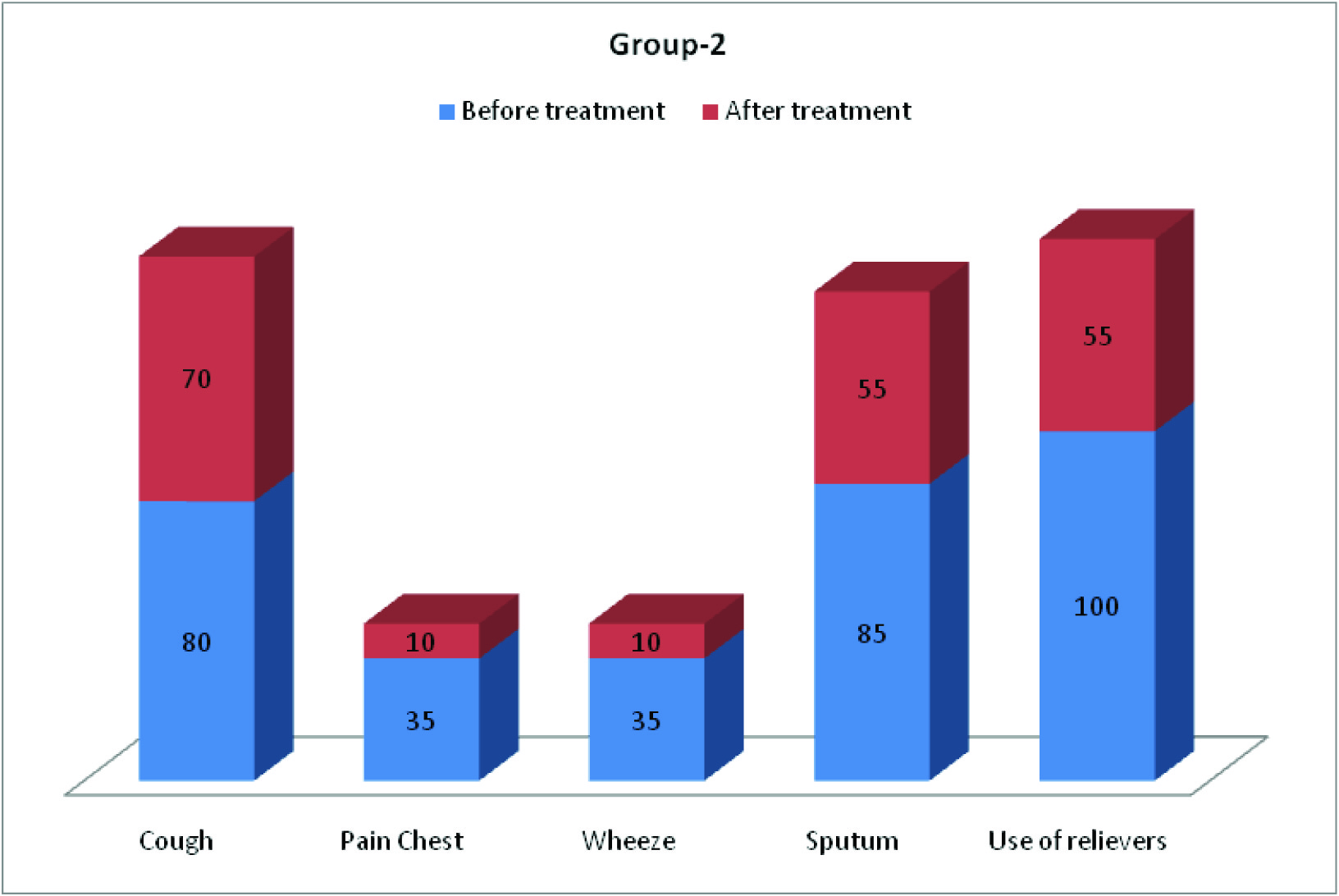
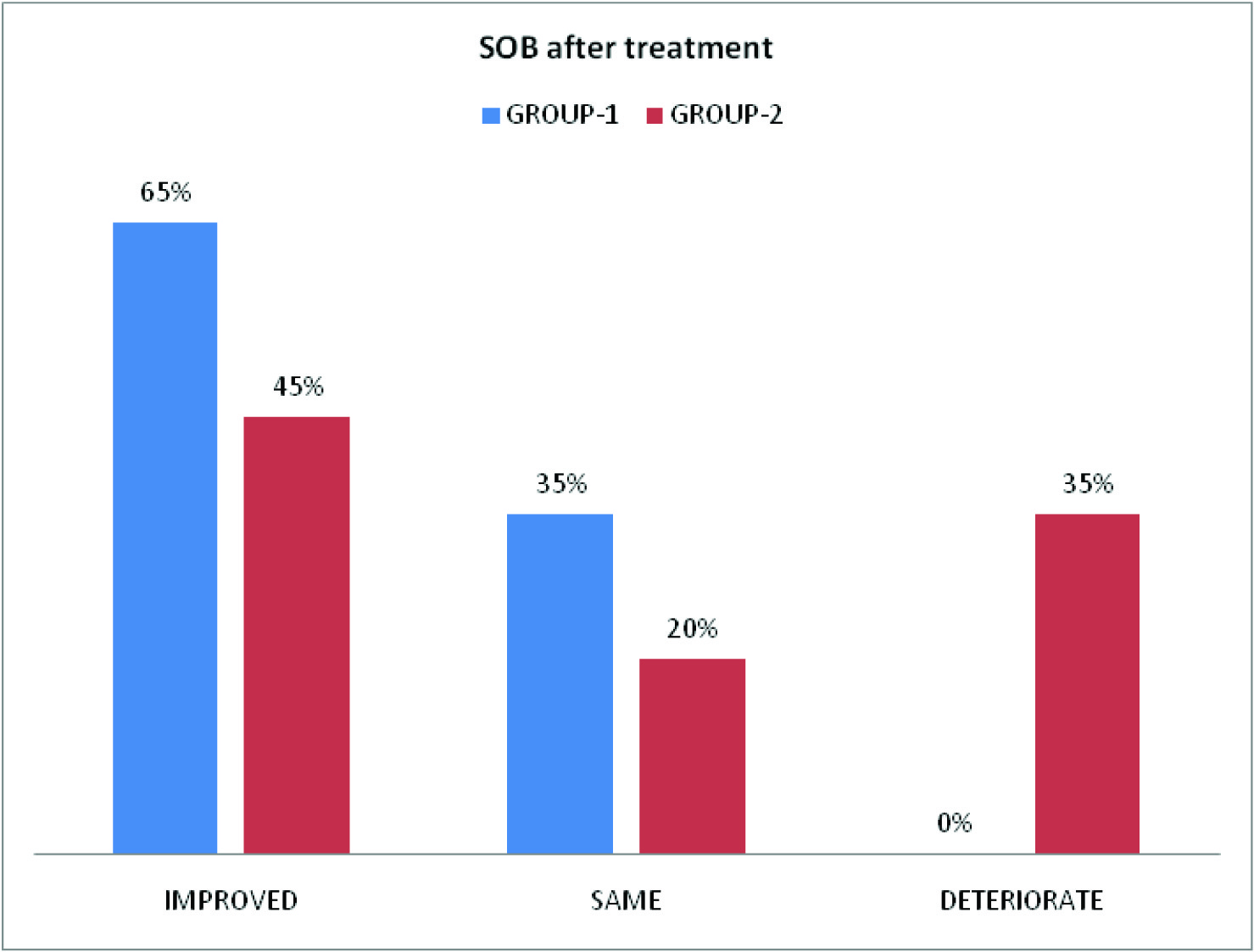
Clinical parameters (cough, pain chest, wheeze, sputum, frequency of use of relievers and SOB) before and after treatment of COPD patients in group-1 and group-2 are depicted in [Table/Fig-6,7,8]. [Table/Fig-8] shows improvement of shortness of breath by 65% in group-1 and 45% in group-2.
Discussion
(FEV1, FEV1/FVC, PEFR, FEF 25-75%) were comparable in two groups (p-value>0.05). There was consistent improvement of spirometric parameters like FEV1, FEV1/FVC ratio and FEF 25-75% in all the three visits of the patients of group-1 (Acebrophylline group), which was statistically significant (p-value<0.05). There was significant change in PEFR, in visit-3 compared to visit-1 (p-value=0.01). These findings are quite similar to Giovanni Agliati [6], E. Pozzi [7]. On the other hand, the patients of group-2 (SR Theophylline group) also showed significant improvement of FEV1 and FEF 25-75% in visit-2 and visit-3 compared to visit-1 (p-value<0.05). Similar findings are reported by Danièle Murciano et al., [8], Donald A Mahler [9]. There was significant change in FEV1 FVC ratio in visit-2 compared to visit-1 (p-value=0.0008). But, no significant change was observed in case of PEFR.
Persistent deterioration in FEV1 and FEV1/FVC is the hallmark of COPD. The progression of COPD is associated with impairment of small airways which is likely related to infiltration of airway walls by inflammatory cells, accumulation of exudate and narrowing of the lumen [10]. With successful management of COPD this pathophysiology may be arrested or improved to some extent. Thus spirometric parameters like FEV1, FEF 25-75% and FEV1/FVC ratio showed improvement with treatment.
On intergroup comparison of PFT parameters, there was no significant change between the two groups and thus were comparable in terms of spirometric improvement.
Symptomatic benefit was observed mainly in three aspects: firstly, there was reduction in the amount of sputum (in 40% patients of group-1 and 55% patients of group-2). Secondly, regarding the frequency of use of reliever medications, 55% of patients of Acebrophylline group and 45% patients of SR Theophylline group showed no need. Thirdly, shortness of breath (according to mMRC scale), was improved in 65% patients of group-1 and 45% patients of group-2. These findings are in agreement with the study by Giovanni Agliati [6], E. Pozzi[7], Danièle Murciano et al., [8], Donald A Mahler [9], and H.Weber [11]. There was no deterioration among patients of Acebrophylline group but breathlessness deteriorated in 35% patients on SR Theophylline.
Regarding the side-effects/tolerability, cardiovascular related complaints e.g. pain chest, palpitation, tremor, tachycardia were not found in patients treated with Acebrophylline. Insomnia and sleep disorder were also absent in them, whereas epigastric tenderness (in 15% of patients), nausea (in 15% of patients), dizziness (in 10% of patients), headache (in 10% of patients) were reported. The reduced occurrence of cardiovascular and CNS side- effects with Acebrophylline may be due to the fact that Ambroxol present in it attains higher concentration in blood than its Xanthine derivative which is associated with untoward side-effects [7]. In patients on SR Theophylline, CNS features like dizziness and headache were absent, whereas cardio vascular related complaints, like pain chest (in 5% of patients), palpitation (in 25% of patients), tremor (in 25% of patients) and tachycardia (in 35% of patients) were present. Insomnia, sleep disorders, epigastric tenderness and nausea were also reported with SR Theophylline.
Showed the base line demographics and PFT parameters in group-1 and group-2 (Mean±SD)
| PARAMETERS | GROUP -1 | GROUP -2 | P-VALUE |
| AGE | 54.75±9.51 | 54.65±9.02 | 0.97 |
| BMI | 21.41±4.41 | 20.25±4.07 | 0.39 |
| FEV1 | 66.28±7.64 | 65.71±7.73 | 0.82 |
| FEV1/FVC | 57.91±9.66 | 57.59±9.46 | 0.92 |
| FEF 25-75 | 40.14±5.81 | 39.83±5.85 | 0.87 |
| PEFR | 46.13±6.43 | 46.01±6.51 | 0.95 |
Showed co-morbidities of patients in two groups
Showed intra-group comparison of PFT parameters.
| PARAMETERS | GROUP -1 (ACEBROPHYLLIN E) | GROUP -2 (SR TH EOPHYLLIN E) |
| FEV1 | Visit-1 | Visit-2 | p-value | Visit-2 | Visit-3 | p-value | Visit-1 | Visit-3 | p-value | Visit-1 | Visit-2 | p-value | Visit-2 | Visit-3 | p-value | Visit-1 | Visit-3 | p-value |
| 66.28±7.64 | 66.75±7.71 | 0.0001* | 66.75±7.71 | 67.52±7.93 | 0.003* | 66.28±7.64 | 67.52±7.93 | 0.0001* | 65.71±7.73 | 65.76±7.86 | 0.304 | 65.76±7.86 | 66.07±8.04 | 0.0005* | 65.71±7.73 | 66.07±8.04 | 0.001* |
| FEV1/FVC | Visit-1 | Visit-2 | p-value | Visit-2 | Visit-3 | p-value | Visit-1 | Visit-3 | p-value | Visit-1 | Visit-2 | p-value | Visit-2 | Visit-3 | p-value | Visit-1 | Visit-3 | p-value |
| 57.91±9.66 | 58.21±9.68 | 0.0001* | 58.21±9.68 | 58.54±9.82 | 0.0005* | 57.91±9.66 | 58.54±9.82 | 0.0001* | 57.59±9.46 | 57.53±9.67 | 0.44 | 57.53±9.67 | 57.75±9.75 | 0.0008* | 57.59±9.46 | 57.75±9.75 | 0.11 |
| FEF25-75% | Visit-1 | Visit-2 | p-value | Visit-2 | Visit-3 | p-value | Visit-1 | Visit-3 | p-value | Visit-1 | Visit-2 | p-value | Visit-2 | Visit-3 | p-value | Visit-1 | Visit-3 | p-value |
| 40.14±5.81 | 40.46±5.75 | 0.008* | 40.46±5.75 | 40.77±5.83 | 0.0002* | 40.14±5.81 | 40.77±5.83 | 0.0001* | 39.83±5.85 | 39.89±5.83 | 0.36 | 39.89±5.83 | 40.08±6.01 | 0.05* | 39.83±5.85 | 40.08±6.01 | 0.02* |
| PEFR | Visit-1 | Visit-2 | p-value | Visit-2 | Visit-3 | p-value | Visit-1 | Visit-3 | p-value | Visit-1 | Visit-2 | p-value | Visit-2 | Visit-3 | p-value | Visit-1 | Visit-3 | p-value |
| 46.13±6.43 | 46.54±6.14 | 0.11 | 46.54±6.14 | 46.7±6.29 | 0.48 | 46.13±6.43 | 46.7±6.29 | 0.01* | 46.01±6.51 | 45.99±6.63 | 0.76 | 45.99±6.63 | 46.44±6.53 | 0.33 | 46.01±6.51 | 46.44±6.53 | 0.36 |
p-value <0.05* was considered to be significantInhaled
Showed inter group comparison of PFT parameters in visit-2 and visit-3.
| PARAMETERS | VISIT -2 | VISIT -3 |
| FEV1 | GROUP-1 | GROUP-2 | p-value | GROUP-1 | GROUP-2 | p-value |
| 66.75±7.71 | 65.76±7.86 | 0.69 | 67.52±7.93 | 66.07±8.04 | 0.57 |
| FEV1/FVC | GROUP-1 | GROUP-2 | p-value | GROUP-1 | GROUP-2 | p-value |
| 58.21±9.68 | 57.53±9.67 | 0.83 | 58.54±9.82 | 57.75±9.75 | 0.79 |
| FEF25-75% | GROUP-1 | GROUP-2 | p-value | GROUP-1 | GROUP-2 | p-value |
| 40.46±5.75 | 39.89±5.83 | 0.76 | 40.77±5.83 | 40.08±6.01 | 0.72 |
| PEFR | GROUP-1 | GROUP-2 | p-value | GROUP-1 | GROUP-2 | p-value |
| 46.54±6.14 | 45.99±6.63 | 0.79 | 46.7±6.29 | 46.44±6.53 | 0.89 |
p-value <0.05 was considered to be significant
Showed side-effects/tolerability (in percentage) in two groups
Showed clinical parameters (in percentage) in group-1 before and after treatment
Showed clinical parameters (in percentage) in group-2 before and after treatment
Showed improvement (%) of SOB in two groups before and after treatment
Recommendations
1. Acebrophylline is a better choice than Theophylline for COPD patients having cardiovascular co-morbidity.
2. Sustained release Theophylline and Acebrophylline are both effective as add-on therapy with Tiotropium (LAMA) in relieving symptoms as well as improving spirometric parameters in moderate COPD patients.
Limitations
1. The issue of observer’s bias could not be eliminated as ‘blinding’ was not done.
2. The benefit of treatment is also attributable to inhaled Tiotropium.
Conclusion
So, it may be concluded that, 1) Methylxanthines are quite effective in the management of moderate COPD as add on therapy to inhaled Tiotropium. 2) Acebrophylline and Sustained release Theophylline are comparable in respect of improvement of spirometric parameters and symptomatic benefit of COPD patients. 3) Moreover it is evident that, Acebrophylline is safer than SR Theophylline in respect of cardiovascular and central nervous system related side effects. These findings also paves the way for further studies in selected group of patients who are suffering from COPD along with some cardiac problems.
p-value <0.05* was considered to be significantInhaled
p-value <0.05 was considered to be significant
[1]. Global Initiative for Chronic Obstructive Lung Disease. The Global Strategy for Diagnosis, Management and Prevention of COPD (Updated 2010). Available fromhttp//www.goldcopd.org/uploads/users/files/GOLDReport April 11 2011 [Google Scholar]
[2]. KF Rabe, S Hurd, A Anzueto, Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. Am. J. Respir. Crit Care Med. 2007 176(6):532-55. [Google Scholar]
[3]. Reilly John J, Silverman Jr. Edwin K., Shapiro Steven D, EH Kass, Chronic obstructive pulmonary disease. Anthony S. Fauci, Eugene Braunwald, Dennis L. Kasper, Stephen L. Hauser, Dan L. Longo, J. Larry Jameson, Joseph Loscalzo. Harrison’s Principles of Internal Medicine. 2008 217th EditionMc Graw Hill:1635-43. [Google Scholar]
[4]. Senior Robert M., Atkinson Jeffrey J., NH Olsen, JJ Hefferren, Chronic Obstructive Pulmonary Disease: Epidemiology, Pathophysiology, and Pathogenesis in Fishman’s pulmonary diseases and disorders. Alfred P. Fishman, Jack A. Elias, Jay A. Fishmans, Michael A. Grippi, Robert M. Senior, Allan I. Pack.J Am Dent Assoc 2008 14th EditionMc Graw Hill:707-27. [Google Scholar]
[5]. J Peter, Barnes. Theophylline: New Perspectives for an Old Drug.Am J Respir Crit Care Med. 2003 167:813-18. [Google Scholar]
[6]. Agliati Giovanni, Acebrophylline in the treatment of chronic obstructive pulmonary disease.Current Therapeutic Research. 1995 56(2):169-75. [Google Scholar]
[7]. Pozzi E., Acebrophylline: an airway mucoregulator and anti-inflammatory agent.Monaldi Arch Chest Dis. 2007 67(2):106-15. [Google Scholar]
[8]. Murciano Danièle, Auclair Marie-Hélène, Pariente René, A Randomized, Controlled Trial of Theophylline in Patients with Severe Chronic Obstructive Pulmonary Disease. N Engl J Med. 1989 320:1521-25. [Google Scholar]
[9]. Donald A Mahler. The role of Theophylline in the treatment of dyspnea in COPD.http://journal.publications.chestnet.org/ Available from on 11/3/2013 [Google Scholar]
[10]. JC Hogg, F Chu, S Utokaparch, The nature of small airway obstruction in chronic obstructive pulmonary disease.N. Engl. J. Med. 2004 350(26):2645-53. [Google Scholar]
[11]. Weber H., Treatment of bronchial asthma- clinical evaluation of a muco-regulating bronchodilator, Ambroxol Theophyllinacetate. Clinical Trials Journal. 1985 22(5):435-40. [Google Scholar]