Periodontitis has multifactorial aetiology with the primary aetiologic agents being pathogenic bacteria that reside in the subgingival area and possess potent mechanisms of damaging host defences. Inflammatory responses triggered in response to periodontal pathogens are the major events responsible for periodontal destruction [1,2].
Treatment of periodontal disease is directed towards the suppression /elimination of subgingival microflora. Root debridement performed by mechanical means, i.e., scaling and root planing (SRP), is the most commonly used initial treatment approach [3]. However, comprehensive mechanical debridement of sites with deep periodontal pockets is difficult to accomplish. It alone, may fail to eliminate the pathogenic microflora because of their location within the gingiva or in areas inaccessible to periodontal instruments [3]. In view of the complex ecosystem within the subgingival pocket, the adjunctive use of antimicrobial agents have been advocated along with mechanical instrumentation to minimize the need for surgical treatment of pockets [4].
Local antimicrobial therapy has the advantage of providing effective concentration of the drug at the site of infection with minimal systemic load and low risk for the emergence of bacterial resistance [3–5]. The clinical use of antibiotics and other antimicrobial agents, as adjuvants for the treatment of periodontitis, has been extensively investigated [4–6]. Recently, special attention has been paid to natural medication, and Indian propolis is one of them which has drawn the attention over a long period of time [7–9].
Propolis is the generic name for a complex resinous mixture collected by honey bees from the buds and exudates of various plants [9]. Once collected, this material is enriched with saliva and enzyme-containing secretions and used in the construction, adaptation, and protection of hives [9]. The medicinal properties of propolis have been investigated previously [10,11].
Hence, the present study was aimed at clinical and microbiological evaluation of the efficacy of subgingivally delivered Indian propolis extract as an adjunct to SRP in the treatment of periodontitis.
Materials and Methods
The randomized, controlled, parallel, double-blind study (clinician and microbiologist) was conducted at Department of Periodontics, Rajarajeswari Dental College and Hospital, Bangalore, India. Twenty patients, 9 males and 11 females, Aged 25 to 50 years (mean age, 35.6 ± 12.2 y) were included in the study after performing the power analysis. The study was conducted from December 2012 to August 2013. Prior to the execution of the treatment, written informed consent was obtained and the treatment procedure was explained to the patient. Ethical clearance was obtained from Institutional Ethical Committee Review Board. The treatment comprised of two groups. Control group, in which the sites were treated by SRP alone and in the test group, the sites were treated by SRP followed by subgingival placement of Indian propolis. Clinical trial was registered at ClinicalTrial.gov and the number assigned was NCT01943877.
Patients who presented with good general health and were diagnosed with chronic periodontitis having minimum of 20 natural teeth with at least one pocket per quadrant with a pocket probing depth (PPD) between 5 and 8 mm were included in the study. Smokers, pregnant and lactating mothers, patients with any other systemic disease like diabetes that can alter the course of periodontal disease progression and treatment were excluded. In every patient, the selected sites were marked and assigned randomly either to the control group or test group. A consort flowchart is presented as [Table/Fig-1].
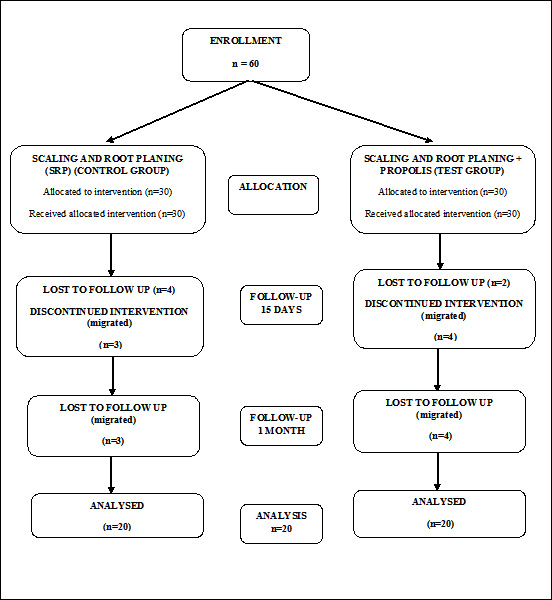
After enrolment by a chief investigator (SPB), sites were randomly assigned to either test or control groups by a flip of a coin. All clinical measurements were performed by a single examiner (NS). On their first visit, all patients were examined in order to register the Gingival index (GI) (Silness and Loe, 1964), Sulcus bleeding Index (BI) (Muhlemann and Son, 1971), Probing pocket depth (PPD) and clinical attachment level (CAL) were measured at six sites per tooth. These parameters were reassessed at 15 days and one month after therapy.
After baseline examination and plaque sample collections, test sites were treated with SRP followed by subgingival administration of propolis, which was preweighed (~5mg) and control sites were treated with SRP alone.
Microbial Sampling
Supragingival plaque was gently removed with sterile cotton pellets and sample sites were isolated with cotton rolls and air-dried prior to sampling. At baseline (prior to SRP) and at 15 days and one month post-treatment, subgingival plaque samples were collected from the deepest portion of the pocket using sterile curettes from test and control sites. Subgingival specimens were collected with a single stroke after gentle insertion into the bottom of the sampling site.
Samples collected in coded sterile vials containing thioglycollate broth were transported to the laboratory for microbial analysis. Once it was received in the laboratory, the sample was mixed thoroughly and was inoculated using sterile loop onto the following medium: Enriched Blood Agar (Porphyromonas gingivalis {Pg}), Brewer’s Anaerobic Agar (Fusobacterium nucleatum {Fn}) and Blood Agar (Prevotella intermedia {Pi}).
Technique for Drug Delivery
A plastic filling instrument was used to carry and place propolis into the test sites, after completion of SRP. The drug was placed such that it was not exposed to the oral cavity. Normal oral hygiene was observed. Patient was advised to avoid proximal cleaning until seven days after treatment of the test sites. Clinical parameters were assessed at 15 days and one month after treatment.
Statistical Analysis
Data analysis was performed using the patient as the experimental unit. The statistical analysis was done using SPSS version 15.0 statistical analysis software. The values were represented in number (%) and mean ± standard deviation (SD). The intragroup comparison was done using Wilcoxon’s signed rank test to evaluate the difference between two treatments or conditions where the samples were correlated and the intergroup comparison was done using the Mann-Whitney U-test to determine if a difference exists between two groups. In the present study p value less than 0.05 was considered as the significant.
Results
Twenty sites were allocated to control group and 20 sites to test group. At no time did any patient experience adverse effects by the use of propolis. No local allergic reaction, pain, swelling, or other side effects were observed throughout the study.
Intragroup evaluation at different time intervals in the test group showed that all the changes were significant statistically at all the time intervals (p < 0.05). Evaluation of change at different time intervals in the control group also showed that the changes were significant statistically at all the time intervals (p < 0.05), as shown in [Table/Fig-2]. A comparison of the mean change in clinical parameters between baseline and one month revealed a statistically significant intergroup difference for all the parameters with the test group showing significantly higher change as compared to the control group (p < 0.05) as shown in [Table/Fig-2].
Comparison of clinical parameters in control and test sites at different time points
| Control Site (SRP) | Test Site (SRP + Propolis) |
|---|
| Baseline (Mean αSD) | 15 DAYS (Mean αSD) | 1 Month (Mean αSD) | Baseline (Mean αSD) | 15 DAYS (Mean αSD) | 1 MONTH (Mean αSD) |
|---|
| Mean GI | 2.05α0.32 | 1.63α0.17 | 1.20α0.22 | 2.04α0.26 | 1.43α0.16 | 0.96α0.09 |
| Mean BI | 2.90α0.45 | 2.20α0.22 | 1.60α0.21 | 2.99α0.32 | 2.15α0.22 | 1.08α0.23 |
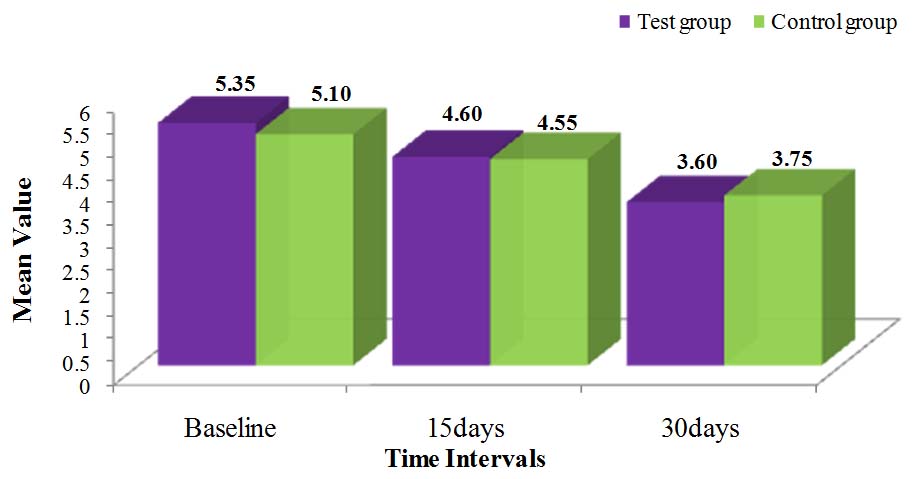
| Mean PPD | 5.10α0.55 | 4.55α0.83 | 3.75α0.79 | 5.35α0.67 | 4.60α0.68 | 3.60α0.68 |
| Mean CAL | 3.10α0.55 | 2.55α0.83 | 1.75α0.79 | 3.35α0.67 | 2.10α0.79 | 1.60α0.68 |
[Table/Fig-2] shows the mean GI, BI, PPD and CAL scores in both test and control sites at different points of time i.e. baseline, 15days and one month. Percentage change seen in clinical parameters in test and control sites at baseline, 15 days, one month is shown in [Table/Fig-3]. [Table/Fig-4] depicts the changes in the mean value of PPD at various points of time.
Percentage change in the clinical parameters in control and test sites at different time points
| Clinical Data | % Change at Control Sites (SRP) | % Change at Test Sites (SRP + Propolis) |
|---|
| Baseline- 15 Days & p-value* | Baseline- 30 Days & p-value* | 15 Days - 30 Days & p-value* | Baseline- 15 Days & p-value* | Baseline- 30 Days & p-value* | 15 Days - 30 Days & p-value* |
|---|
| GI | 20.73 0.0002 | 41.46 0.0001 | 26.15 0.0001 | 30.06 0.0001 | 52.76 0.0001 | 32.46 0.0001 |
| BI | 24.14 0.0001 | 44.83 0.0001 | 27.27 0.0001 | 28.03 0.0001 | 64.02 0.0001 | 50.00 0.0001 |
| PPD | 10.78 0.0033 | 26.47 0.0001 | 17.58 0.0010 | 14.02 0.0007 | 32.71 0.0001 | 21.74 0.0001 |
| CAL | 17.74 0.0033 | 43.55 0.0001 | 31.37 0.0010 | 37.31 0.0003 | 52.24 0.0001 | 23.81 0.0001 |
* Comparison between groups (Mann-Whitney U test); p<0.05
Comparison of test and control groups with respect to probing pocket depth scores at baseline, 15 days and 30 days
Microbiological Analysis
Subgingival plaque samples for microbiological analysis were taken at the test and control sites at baseline, 15 days and one month. Microbiological parameters included evaluation of reduction of the three periodontal pathogens at baseline, 15 days and one month follow-up visits. The quantities of colonies are expressed as colony forming units per ml (CFU/ml). [Table/Fig-5] shows the mean values of colony count/ml for Porphyromonas gingivalis (Pg), Prevotella intermedia (Pi) and Fusobacterium nucleatum (Fn) in test and control groups at different time intervals. At the follow-up intervals, an intragroup comparison revealed a statistically significant (p < 0.05) reduction in both the groups, with the test group showing lower prevalence of all the three microorganisms as compared to the control group.
Comparison of prevalence of Porphyromonas gingivalis, Prevotella intermedia, Fusobacterium nucleatum different time points in test and control sites
| Control Site (SRP | Test Site (SRP + Propolis) |
|---|
| Baseline (Mean αSD) | 15 DAYS (Mean αSD) | 1 Month (Mean αSD) | Baseline (Mean αSD) | 15 DAYS (Mean αSD) | 1 MONTH (Mean αSD) |
|---|
| *Pg | 43.30 ±11.65 | 20.20 ± 9.31 | 7.80 ±5.03 | 43.55 ±13.74 | 15.15 ±3.65 | 4.90 ±3.35 |
| *Pi | 80.35 ±12.93 | 45.90 ±14.85 | 26.25 ±11.22 | 63.75 ±13.36 | 30.20 ±12.17 | 15.15 ±5.47 |
| *Fn | 68.65 ±10.52 | 50.50 ±18.70 | 27.80 ±10.68 | 64.00 ±9.55 | 41.00 ±7.88 | 19.85± 6.80 |
*Pg- Porphyromonas gingivalis; Pi- Prevotella intermedia; Fn- Fusobacterium nucleatum
[Table/Fig-5] shows the mean values of the prevalence of Porphyromonas gingivalis (Pg), Prevotella intermedia (Pi), Fusobacterium nucleatum (Fn) at different time points (baseline, 15 days and one month) in test and control sites. The percentage change of prevalence of Pg, Pi, Fn at baseline, 15 days and one month is shown in [Table/Fig-6] for both test and control groups.
The percentage of change of prevalence of Porphyromonas gingivalis, Prevotella intermedia, Fusobacterium nucleatum at different time points in test and control sites
| Clinical Data | % Change at Control Sites (SRP) | % Change at Test Sites (SRP + Propolis) |
|---|
| Baseline- 15 Days & p-value* | Baseline- 30 Days & p-value* | 15 Days - 30 Days & p-value* | Baseline- 15 Days & p-value* | Baseline- 30 Days & p-value* | 15 Days - 30 Days & p-value* |
|---|
| *Pg | 53.35 0.00001 | 81.99 0.00001 | 61.39 0.00001 | 65.21 0.00001 | 88.75 0.00001 | 67.66 0.00001 |
| *Pi | 42.87 0.00001 | 67.33 0.00001 | 42.81 0.00001 | 52.63 0.00001 | 76.24 0.00001 | 49.83 0.00001 |
| *Fn | 26.44 0.0002 | 59.50 0.00001 | 44.95 0.00001 | 35.94 0.00001 | 68.98 0.00001 | 51.59 0.00001 |
* Comparison between groups (Mann-Whitney U-test); *p < 0.05.
†Pg- Porphyromonas gingivalis; Pi- Prevotella intermedia; Fn- Fusobacterium nucleatum
Overall, both the groups showed statistically significant reduction in relation to the clinical parameters. Also, the groups showed reduction in the microbial count of Porphyromonas gingivalis, Prevotella intermedia and Fusobacterium nucleatum.
Discussion
The aim of the present study was to compare the clinical and microbiological benefits of routine mechanical therapy with adjunctive use of Indian propolis as a local drug delivery (LDD) agent in the treatment of periodontitis. LDD agents are available for use as adjuncts to SRP. They reduce the subgingival bacterial flora and the clinical signs of periodontitis. LDD may be used as an adjunct to SRP when localised recurrent and/or residual probing depth of ≥ 5 mm with inflammation is still present after conventional therapies [18].
Complementary and alternative medicine (CAM) represents a group of diverse medical and health care systems, practices, and products that are not considered to be part of conventional medicine [19]. Biofeedback, acupuncture, herbal medication, massage, bioelectromagnetic therapy, meditation, and music therapy are examples of CAM treatments. Apitherapy, or therapy with bee products (e.g. honey, pollen, propolis, fortified honey, etc) is an old tradition that has been revitalized in recent research [20]. Natural products like propolis are preferred due to lesser side effects and lower cost.
Propolis is highly regarded for its medicinal properties [7,11–17]. The antimicrobial properties of propolis against human pathogens have been known since antiquity [6]. Previously, Santos et al., Feres et al., and Koru et al., confirmed antibacterial properties of propolis in relation to pathogens of periodontitis [21–24]. Santos et al., also indicated that antibacterial effects are conditioned by flavonoids, phenol acids and their esters [21].
In the present study, the efficacy of propolis as a local drug delivery was evaluated over SRP alone for a period of one month. The clinical parameters were recorded at 1 month as the bacterial flora is known to return to pre-treatment patterns after 3-6 weeks of SRP [22].
Significant reduction in the GI was seen in the test group and the control group from baseline to 1 month with the test group showing significantly higher reduction (0.96±0.09) as compared to the control group (1.20±0.22) (p < 0.001). Similarly, a mean reduction in BI was significantly higher in the test group (1.53 ± 0.52) as compared to the control group (0.67 ± 0.41) (p < 0.001) as shown in [Table/Fig-3]. These findings are probably justified by the antibacterial and anti-inflammatory effects of propolis. This is in correlation with a recent study in which propolis was used an alcohol-free mouthwash containing 5.0% (W/V) Brazilian green propolis for the control of plaque and gingivitis [14]. Twenty five participants were instructed to rinse with 10ml of mouthwash test for one minute, immediately after brushing in the morning and at night. After 45 and 90 days of using mouthwash, the results showed a significant reduction in plaque and in gingival index when compared to that at baseline [14].
Research done by Coutinho made it possible to conclude that additional subgingival irrigation with a propolis extract during periodontal treatment allowed to obtain better results than SRP by themselves, which results from the assessment of both clinical and microbiological parameters [15]. The results of our study correlated with the aforementioned research.
At baseline, the majority of the evaluated sites exhibited high levels of P gingivalis, P intermedia and F nucleatum. The use of propolis in test sites showed significant reduction in the prevalence of the three pathogens. Some in vitro and in vivo studies have demonstrated activity of propolis against periodontal pathogens [7,17,21–24,25–28]. Mechanisms of activity of propolis against microorganisms are still controversial. Some components present in propolis extracts like flavonoids (quercetin, galangin, pinocembrin) and caffeic acid, benzoic acid, cinnamic acid, probably act on the microbial membrane or cell wall site, causing functional and structural damages [27].
Propolis has anti-inflammatory effects and it acts by modulating the cytokines and inflammatory mediators, such as suppression in the production of prostaglandins, histamine, TGF-β[17]. The results of use of propolis on the severity of gingival and bleeding indices scores suggest their anti-inflammatory effects [16]. Evidence suggests that propolis may actively protect against oral disease due to its antimicrobial properties [17,28]. Because of its strong, anti-infective activity, propolis has often been called a “natural antibiotic.”
There was no adverse effect seen in any of the patients treated with propolis in the present study. The varied benefits of propolis, such as affordability, easy availability, antibacterial and antiinflammatory properties make propolis a potential therapeutic agent in periodontal therapy. The limitations of the present study include larger sample size, use of more sensitive microbiological analysis techniques like polymerase chain reaction.
Conclusion
Our study demonstrated reduction of GI, BI, PPD, CAL in the test group treated with scaling and root planing and propolis when compared to the control sites treated with SRP alone. Subgingivally delivered propolis as an adjunct to scaling and root planing in the treatment of chronic periodontitis has shown promising results. Further research focussing on the in vivo and in vitro anti-inflammatory/antimicrobial effects of propolis done on larger sample size is required for the better understanding of its precise role in the treatment of periodontitis. It is therefore comprehensible that we should now focus on “back to nature approach” where propolis seems to be a promising alternative for the control of oral diseases in terms of antimicrobial properties and lower associated risks.
* Comparison between groups (Mann-Whitney U test); p<0.05
*Pg- Porphyromonas gingivalis; Pi- Prevotella intermedia; Fn- Fusobacterium nucleatum
* Comparison between groups (Mann-Whitney U-test); *p < 0.05.
†Pg- Porphyromonas gingivalis; Pi- Prevotella intermedia; Fn- Fusobacterium nucleatum