Aim: Migraine headache is a common disorder, several drugs have been tried as a prophylaxis to reduce the attacks of headache. Aim of this study is to see the impact of amitriptyline on quality of life in migraneurs using Migraine Disability Assessment Score.
Materials and Methods: In this study 300 patients of either gender who required prophylaxis for migraine without aura were selected. The patients were of the age group 18-60 years. The MIDAS questionnaire was administered to patients before starting treatment and again after 45 days prophylaxis. The improvement was noted.
Statistical Analysis: It is a prospective study where the severity of symptoms and ‘quality of life’ in patients is assessed before and after treatment using mean, frequency, standard deviation and paired‘t’ test.
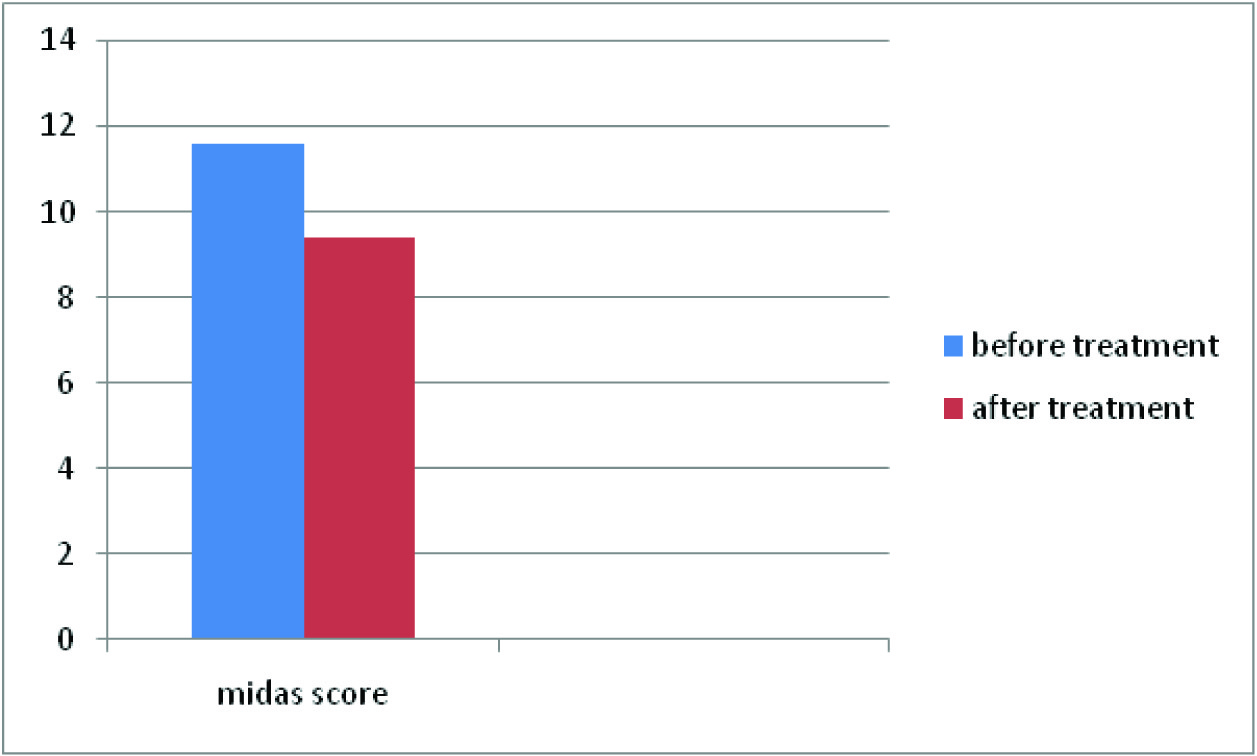
Results: A total of 300 patients were subjected to migraine prophylaxis with amiptriptyline. The study showed female preponderance. The mean MIDAS score before and after treatment with amitriptyline was 11.6 and 9.4 respectively. The student‘t’ test showed p-value of <0.005 which was significant.
Conclusion: Migraine prophylaxis with amitriptyline for a period of 45 days decreased the severity of symptoms and also reduction in days of migraine attacks. However the long term effects on quality of life could not be assessed. The study needs to be conducted in a large scale to evaluate the consistency and accuracy of the test.
Introduction
Migraine headache is a complex, recurrent headache disorder that is one of the most common complaints in otorhinolaryngology. Migraine is a common disabling primary headache disorder. In the United States, around 30 million people have 1 or more migraine headaches per year. This amounts to approximately 18% females and 6% males [1] . Approximately 75% of all migraine sufferers are women. The term migraine takes its origin from Greek word hemikrania. This term was translated in the Latin as hemigranea, which means migraine in French. According to the WHO, migraine is 19th among all causes of years lived with disability. It is defined as per ICHD-2 diagnoses. Migraine can be divided into two major sub-types.
1) Migraine without aura is a clinical syndrome characterised by headache with specific features and associated symptoms.
2) Migraine with aura is primarily characterized by the focal neurological symptoms that usually precede or sometimes accompany the headache [2].
Migraine without aura [3].
Recurrent headache disorder presenting with headache attacks lasting 4–72 hours. The headaches are unilateral in location, pulsating quality, moderate or severe intensity, aggravated by routine physical activity and associated with nausea, photophobia and/or phonophobia.
Diagnostic criteria
At least 5 attacks, headache attacks lasting 4–72 hours, headache has at least two of the following characteristics - unilateral location, pulsating quality, moderate or severe pain intensity, aggravation by or causing avoidance of routine physical activity [Table/Fig-1] .
During headache at least one of the following:- Nausea and/or vomiting, Photophobia and phonophobia. Hu and colleagues [4] showed that early treatment of an acute attack was associated with an overall reduced migraine burden for the event, and early treatment is commonly emphasized in clinical recommendations.
Criteria for prophylaxis
If patient has 3 or more severe migraine attacks per month which do not respond adequately to abortive or symptomatic therapy, the migraine attacks are severe enough to affect the quality of life, patient who has > 2 headaches/week that require pharmacologic intervention.
Few medications for prophylactic treatment have been subjected to adequate clinical trials. Good response may be defined as 50% reduction in the frequency or severity of migraines. Underlying principle is to use the least amount of medication with the fewest side effects to gain control of symptoms until the preventive treatment can be stopped. The medication should be continued for an adequate period, usually several months, and withdrawn slowly to prevent medication overuse headaches. Prophylactic treatment requires a major commitment by both the patient and the prescriber. T.C.A falls in level 2, B drugs used for migraine prophylaxis as per-The Clinical Practice Guidelines for Adult Migraine Assessment and Treatment. The MIDAS or Migraine Disability Assessment Test is a test used by doctors to determine how severely migraines affect a patient’s life. Patients are asked questions about the frequency and duration of their headaches, as well as how often these headaches limited their ability to participate in activities at work, at school, or at home. The test was evaluated by the professional journal Neurology in 2001; it was found to be both reliable and valid [5].
This study aimed to evaluate the impact of Amitriptyline on MIDAS score.
Source of the Data
The study was conducted in the Department of Otorhinolaryngology, Fr. Muller Medical College, Mangalore, India.
Materials and Methods
The study was done on 300 patients of either gender, age group 18-60, who required prophylaxis for migraine without aura.
Inclusion Criteria: Those who had 3 or more severe migraine attacks per month that failed to respond to abortive therapy, migraine attacks was severe enough to impair the patient’s quality of life, patient who had > 2 headaches/week that required pharmacologic interventions were included.
Exclusion Criteria: Acute recovery phase following MI, and with concomitant use of monoamine oxidase inhibitors, urinary retention, seizure disorder, glaucoma, hyperthyroidism, schizophrenia, pregnancy and lactation period were excluded.
Demographic data like age, sex were recorded. The MIDAS questionnaire [6,7] was administered to patients before starting treatment and again after 45 days prophylaxis. The improvement was noted.
Developed by Dr Lipton, professor of neurology, Albert Einstien college of medicine New York
| Midas grade | Definition | Midas score |
| MIDAS Grade I | Little or no disability | 0-5 |
| MIDAS Grade II | Mild disability | 6 to 10 |
| MIDAS Grade III | Moderate disability | 11 to 20 |
| MIDAS Grade IV | Severe disability | 20+ |
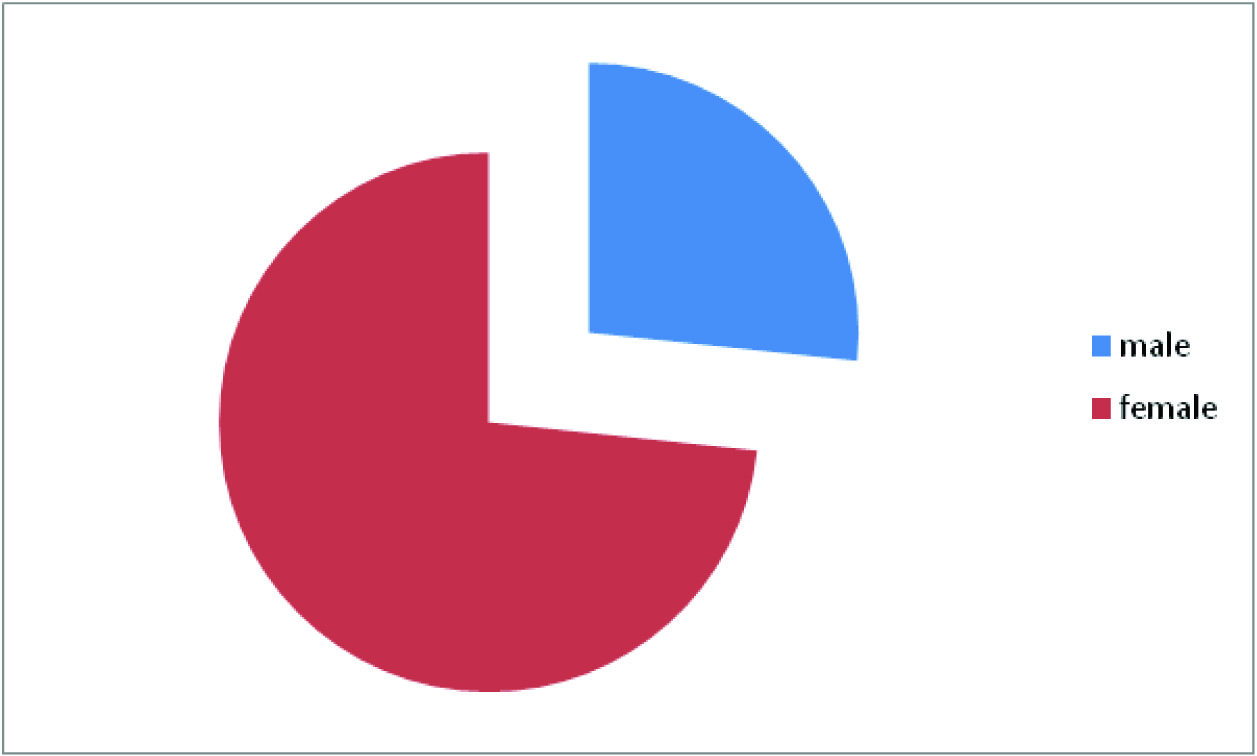
Male and female distribution shows female preponderance 2.75: 1
The mean MIDAS score before the initiation of the treatment was 11.6 and the score post treatment with Amitriptyline was 9.2
Statistical Analysis
It is a prospective study where the severity of symptoms and ‘quality of life’ in patients is assessed before and after treatment using mean, frequency, standard deviation and paired t-test [Table/Fig-1] .
Results
The present study showed out of 300 patients 220 were females and 80 were males. 70% of the females and 87.5% of males were in the age group of 30-40 years [Table/Fig-2]. The mean duration of the symptoms was 3 months. 5% of the female population did not show any improvement. The mean MIDAS score before the initiation of the treatment was 11.6 and the score post treatment with Amitriptyline was 9.2 [Table/Fig-3].
Discussion
The female-to-male ratio increases from 2.5:1 at puberty to 3.5:1 at 40 years of age. In our study 70% of the females were in the age group of 30- 40 years. The mean MIDAS score before and after treatment with Amitriptyline was 11.6 and 9.4 respectively.In a study conducted by Jay Erickson [8] on 365 soldiers, thenumber of headache days reduced from 11.6 days to 6.1 days ina subset of soldiers who received Amytriptyline. Study conductedby Buchanan and colleagues [9] suggest that Amitriptyline can behelpful in migraine patients with associated sleep disruption, oftendescribed as a fragmented, discontinuous sleep pattern, becauseit can stabilize sleep. The student ‘t’ test showed p-value of <0.005which was significant. A controlled clinical trial conducted byRodriguez et al., [10]on migraine prophylaxis showed that thefrequency of migraine episodes came down drastically from 272to 87 after receiving Amitriptyline prophylaxis for 4 months duration(1mg/kg/day). It was also noted that Amitriptyline did not reduce the intensity of the migraine episodes significantly. David et al., [11]conducted a study on migraine prophylaxis with Amitriptyline usinga different scale (Migraine Specific quality of life Questionnaire).Results showed significant (p=0.04) reduction in mean functionaldisability score during migraine attacks. Similar studies on Topiramateshowed much better reduction in days of migraine attacks frombase line [12]. To our study a prophylaxis of a minimum period of3 months would have yielded consolidating results. Certain lifestylemodifications like avoidance of exposure to sun light, loud noise,chocolate, tea and coffee, good sleep hygiene and regular foodhabits could have played a role in improving the quality of life buttheir role could not be evaluated.
Conclusion
Migraine prophylaxis with amitriptyline for a period of 45 days decreased the frequency of symptoms and also reduction in days of migraine attack thus improving the quality of life in migraineurs.
[1]. RB Lipton, AI Scher, K Kolodner, J Liberman, TJ Steiner, WF Stewart, Migraine in the United States: epidemiology and patterns of health care use.Neurology. 2002 58(6):885-94. [Google Scholar]
[2]. JN Blau, Migraine prodromes separated from the aura: complete migraine.BMJ. 1980 281:658-60. [Google Scholar]
[3]. RJ Eapen, CS Ebert, C Harold, Pillsbury H C.Allergic Rhinitis History and Presentation.Otolaryngol Clin N Am. 2008 41:325-30. [Google Scholar]
[4]. XH Hu, D Ng-Mak, R Cady, Does early migraine treatment shorten time to headache peak and reduce its severity?Headache. 2008 48(6):914-20. [Google Scholar]
[5]. WF Stewart, Development and testing of the Migraine Disability Assessment (MIDAS) Questionnaire to assess headache-related disability.Neurology. 2001 56:S20-28.(6 Suppl 1) [Google Scholar]
[6]. http://uhs.berkeley.edu/home/healthtopics/pdf/assessment.pdf [Google Scholar]
[7]. WF Stewart, RB Lipton, KB Kolodner, J Sawyer, C Lee, JN Liberman, Validity of the Migraine Disability Assessment (MIDAS) score in comparison to a diary-based measure in a population sample of migraine sufferers.Pain. 2000 88(1):41-52. [Google Scholar]
[8]. Erickson Jay, A Pilot Randomized Controlled Trial of Prophylactic Medications for Chronic Post-Traumatic Headaches in U.S. Army Soldiers.Neurology. 2012 78:03-226. [Google Scholar]
[9]. TM Buchanan, NM Ramadan, Prophylactic pharmacotherapy for migraine headaches.Semin Neurol. 2006 26:188-98. [Google Scholar]
[10]. Ildefonso Rodríguez-Leyva, Martín Sánchez-Aguilar, Francisco Hernández-Sierra Juan, B Mandeville Peter, Rocío Rodríguez Leyva Ma. del, Manue Shiguetomi-Medina Juan, Topiramate vs. Amitriptyline in prophylactic treatment of migraine: A controlled clinical trial.Revista Mexicana de Neurociencia. 2010 11(5):338-42. [Google Scholar]
[11]. Dodick David W., Freitag Fred, Banks James, Saper Joel, Xiang Jim, Rupnow Marcia, Topiramate Versus Amitriptyline in Migraine Prevention:A 26- Week, Multicenter, Randomized, Double-Blind, Double-Dummy, Parallel-Group Noninferiority Trial in Adult Migraineurs.Clinical Therapeutics. 2009 31(3) [Google Scholar]
[12]. T. Ninan, MD Mathew, A. Sayyed Farhan, MD Jaffri, A Double-blind Comparison of OnabotulinumtoxinA (BOTOX®) and Topiramate (TOPAMAX®) for the Prophylactic Treatment of Chronic Migraine: A Pilot Study.Headache. 2009 49(10):1466-78.http://www.medscape.com/viewarticle/715729_5 [Google Scholar]