Introduction
Head and neck neoplasia is a major form of cancer in India, which accounts for 23% of all cancers among males and 6% among females [1].Oral squamous cell carcinoma (OSCC) accounts for 24% of all the head and neck cancers and 90% of all oral cancers [2]. It has a bizarre incidence worldwide and a brutal prognosis, which lead further research on factors that might modify disease outcome [3].
Factors affecting the prognosis of oral cancer are based on tumour, node and metastasis (TNM) classification which stages the patient according to the size of the primary tumour and the presence of loco-regional and distant metastases. Other prognostic histopathological information includes tumour depth, grade and surgical margin status, as well as cohesiveness (pattern of invasion) and the presence of perineural or lymphovascular invasion [4]. Complete surgical resection is the most important prognostic factor, as the main cause of patient death is incomplete removal of primary tumour [2]. Although, the histopathologic status of the resection margins has long been used as a potential indicator of local recurrence and survival, there is still considerable uncertainty concerning many aspects of resection margins including their nomenclature and definition, and the influence of anatomical and histological factors [5].
Methods for Evaluation of Surgical Margins
There are several techniques to improve the assessment of the marginal extent of the tumour front and subsequently the adequacy of the excision of the primary tumour. Depending on the period of usage they are broadly categorised into the three types.
Working Classification
Preoperative assessment
Vital tissue staining
Flourescent visualisation
II. Intraoperative assessment
Thickeness of resected margins
Frozen section analysis
Touch imprint cytology (TIC)
Microendoscope
Optical coherence tomography (OCT)
III. Postoperative assessment
Moh’s Microsurgery
Frozen technique (Intraoperative assessment)
Formalin technique (Postoperative assessment)
Ultrasonography
Molecular assessment
Gene signature
I. Preoperative
1. Vital Tissue Staining
In vivo staining application, for the detection of malignant change of the cervix during colposcopy is being used extensively in gynaecological practice . This technique can also be applied to the oral setting using various dyes [6].
a) Toluidine blue: This basic metachromatic stain has affinity towards DNA and RNA . Its efficacy has been proven in demonstrating invasive malignancy, carcinoma-in-situ and dysplasia by staining abnormal tissues blue. Therefore, it is used to improve marginal control in the resection of the primary tumour. Moreover, it is useful to define the superficial tumour borders, especially possible malignant or premalignant cells in the surrounding area of the tumour. Hence, toluidine blue has been widely utilised in the clinical routine program of presurgical examinations after the detection of a malignancy of the oral cavity [7].
b) Iodine: Vital staining with an iodine solution has been used widely for the detection of malignant changes of the cervix uteri and the esophagus. Principle of Iodine solution is, it is retained in normal nonkeratinised squamous epithelium and not retained in dysplastic or malignant epithelium. Clinically, the extent and the borders of the intraepithelial lesion, especially in those erythroplakic lesions can be more sharply defined with an iodine solution [7].
c) Indigo carmine and congo red: Indigo carmine and Congo red help in demonstrating the extent or border of tumour invasion. Indigo carmine is a conventional contrast dye used for accentuation of the intestinal mucosa and Congo red is a reactive pH-dependent coloring agent that identifies acid-secreting gastric cells. A consecutive application of 0.4% Indigo carmine and 0.5% of Congo red produces brown-black stain on normal muscle, fibrous/scar, salivary, and most part of adipose tissue excluding invading tumor, enabling us to demarcate the extent of the tumor invasion. The method cannot detect microscopic nests of infiltrating carcinoma and cannot clarify the difference between the invaded carcinoma and scattered adipose tissue, but has the ability to suggest deep surgical margins and to select a site for additional frozen-section assessment [8].
d) Indocyanine green: It is used to stain brain tumours. It is injected intravenously into tumour-bearing rats and was found to stain the tumour intensely at 60 to 120 mg/kg for at least 1h after injection [9].
2. Flourescent Visualisation (FV)
FV device is a visual aid that facilitates the detection of autofluore–scence loss in both visible and occult high-risk oral lesions through direct fluorescence visualisation. Under direct FV, the normal oral mucosa emits various shades of pale green autofluorescence. Clinical lesions that retain the normal green autofluorescence under FV are defined as FV retained (FVR). Tissue which shows a reduction in the normal pale green and appears as dark patches are classified as FV loss (FVL) [10].
Principle: The interaction of light with tissue has ability to highlight changes in the structure and metabolic activity of the areas that are optically sampled.
Mechanism: The loss of autofluorescence is believed to reflect a complex mixture of alterations to intrinsic tissue fluorophore dis–tribution, such as the breakdown of the collagen matrix and a decrease in flavin adenine dinucleotide concentration due to tissue remodeling and increased metabolism associated with neoplastic development. Such changes during neoplastic development will lead to increased absorption and/or scattering of light, thereby reducing and modifying the detectable autofluorescence [10].
Fluorescence and reflectance spectroscopy could be a valuable tool for examining the superficial margin status of excised breast tumour specimens, particularly in the form of spectral imaging to examine entire margins in a single acquisition [11]. Hence, direct FV can identify subclinical high-risk fields with cancerous and precancerous changes in the operating room setting.
II Intraoperative Assessment
1. Thickness of resected margins
In the literature, close surgical margins less than 3 mm or 5 mm have been reported to be associated with a high risk of cancer recurrence. However, there is still no universally agreed definition of close surgical margin [12]. Surgical margin affects locoregional control in such a way that, narrower the surgical margin, the greater the difference in locoregional control after treatment. Patients with surgical margin ≤3 mm had a statistically significantly higher risk for locoregional failure than those with surgical margin more than 3mm [12]. Recurrence rates for patients with margins of 3 to 4 mm were identical to those observed for patients with margins of 5mm [13]. Acc. to Wong LS et al a margin of ≥5mm – clear,1–5mm – close, <1mm – involved [14].
2. Frozen section analysis
It is a valuable intraoperative guide in the management of SCC as it helps to make prompt therapeutic decision that may prevent surgical reintervention or postoperative radiotherapy. A tumour-free resection surface does not guarantee that local recurrence will not occur [15]. But according to Olson TP [16] intraoperative frozen section analysis allows resection of suspicious or positive margins, thereby resulting in low rates of local recurrence and re-excision. This method is routinely used for evaluation of soft tissue margins of resection for head and neck malignancies. But this method applicability for evaluation of cortical bony margins of high density bone is difficult. Typically, it cannot be confirmed until decalcification is completed at 7 to 10 days postoperatively. Oxford LE et al., introduced a method of accurate frozen section analysis of cancellous bone margins in which curved osteotome can be used to obtain thin sections of bony margins.
Procedure: The ostetome has to be directed parallel to the cut surface of the bone to get thin bony sections. Cortical specimens should be thin enough to be translucent. The lower density of cancellous bone typically results in small thin fragments of bone that can be easily collected. The bony margins are then processed by routine frozen section method. A standard cryostat and microtome should be used to prepare slides for staining. Advantage of this method is no requirement of decalcification.
3. Touch Imprint Cytology
Most non cytological methods assess only 10–15% of the surface of the lesion. So, touch preparation cytology can also be one of the methods to evaluate surgical margins.
Principle: Tumour cells if present will adhere to the slide [17].
Method: This method involves simply touching the specimen on to a glass slide. Margins have to be examined first by obtaining touch imprint from six margins. Subsequently margins have to be inked and serial sectioning at 0.5cm intervals have to be taken. Smears have to be prepared and fixed with 95% methanol. Slides are then stained and examined for cytological features of malignancy. Diagnostic categories can be:
negative for carcinoma
atypical cells present but nondiagnostic of carcinoma
atypical cells present
atypical cells present suspicious for carcinoma
positive for carcinoma [18].
Simple, quick (2 to 3 min)
safe (no loss of diagnostic material)
Less expensive
Possible to survey the entire surface area of the lesion (especially during lumpectomy).
4. Microendoscope: Microendoscope is considered as “gold standard” in the determination of surgical margins at operation. It was first popularised by Hamou in 1979 as a technique to study epithelial cells of the uterus. The instrument endoscope was later modified by Andreas [20] for using in the upper aero-digestive tract.
Applications
Invivo examination of the epithelium.
Monitoring of the whole mucosal surface both normal and pathological, and
Allows the detection of patterns specific for pathology e.g. inflammation, metaplasia, dysplasia, and malignancy.
Endoscopes are available in different sizes and with different angulations based on their usage in different anatomical regions [Table/Fig-1].
Diameters and angulation of endoscopes and their applications
| Diameter | Applications |
|---|
| Longer larger endoscopes (diameter 5.5mm, length 23cm) | oro-laryngeal lesions |
| Shorter microendoscopes (diameter 4mm, length 18cm) | oro-nasal lesions |
| Angulations |
| 0° | Medially placed lesions(that could be directly approached with occlusive contact) e.g.: floor of mouth, tongue, inferior turbinate, vocal folds |
| 30° (forward oblique) | Laterally placed lesion e.g.: retro-molar trigone, lateral border of the tongue, buccal sulcus, nasal cavity. |
Methodology: The microendoscope has a fitted rotating screw which allows magnification to be changed from 0x to 60x to 150x. Minor movements help in focussing and de-focussing at specific depths of field. The generalised area has to be surveyed at 0x magnification, until an area of interest is found; that will then be examined at 60x and then 150x. Examination should always proceed from an area of normality to abnormality; the whole of the surface of the lesion has to be reviewed to determine any heterogeneity. The margin of the lesion can then be delineated.
The active migration of cells towards the surface epithelium during maturational turn-over suggests that microendoscopic examination of the surface epithelium can detect underlying mucosal pathology. The disadvantage of this technique are that the microendoscope does not provide direct three dimensional information concerning depth of invasion [20].
High-resolution confocal optical images can be obtained in a non-invasive manner, reveals morphologic details with similar quality to that seen in histological slides, without the need for slide preparation and staining. However, despite their utility and impressive resolution, confocal imaging devices are complex and expensive. An alternative approach is a portable high-resolution microendoscope (HRME), which utilises a flexible fiberoptic probe to examine tissue treated with a topically applied fluorescent nuclear contrast agent. For images of the oral cavity, pharynx, and larynx, areas of interest, including grossly normal tissue, tumour, has to be stained with proflavine. [Table/Fig-2] shows HRME images of benign and malignant mucosa.
Features of benign and malignant squamous epithelium
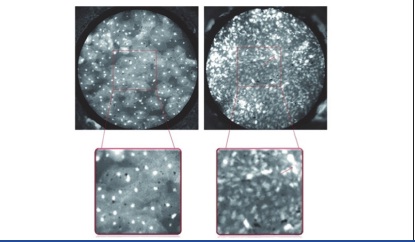
Applications
To identify Barrett’s dysplasia in the esophagus
Axillary lymph node metastasis in breast cancer
Neoplasia in resected oral squamous carcinoma specimens with high sensitivity and specificity [21].
Benign (left) and malignant (right) epithelium from the surface of the tonsil. The boxed area is magnified to display differences in nuclear size, density, and pleomorphism. Note that nuclei in benign tissue are small, punctuate dots that are similarly sized and evenly spaced, while malignant nuclei are larger (red arrow), pleomorphic and irregularly spaced with crowding and loss of normal architecture. Courtesy Levy L Let al., [21].
5. Optical Coherence Tomography
Optical coherence tomography (OCT) is an imaging modality that uses light to determine cross-sectional anatomy in turbid media such as living tissues [22]. The OCT system consists of a super-luminescent diode (SLD), with an optical spectrum. Light is passed through an optical circulator and into a 95/5 fiber-optic splitter that divides the light into a sample and reference arm. Reflected light from the sample and reference arms is passed through polarisation controllers, coupled into an interferometer, spectrally dispersed by a diffraction grating, and focused onto an Indium Gallium Arsenide (InGaAs) line camera. The data is assembled and displayed as a 2-D image. Approximately 8-9 images per second will be obtained by the imaging system [23].
Full field-OCT (FF-OCT) is the first ultra-high resolution optical imaging technique that produces images of high resolution. It directly captures “en face” images on megapixel cameras at high lateral resolution by using medium to large aperture micro–scope objectives and high axial resolution. FF-OCT can image unprocessed tissue samples down to a few hundred microm–eters below the specimen surface and generate 3D image. [Table/Fig-3] shows differences between regular OCT and FF-OCT.
Differences between conventional OCT and FF-OCT
| FF-OCT | OCT |
|---|
| Clear images | Images appear fuzzy |
| Uses immersion microscope objectives with a numerical aperture of 0.3 | Numerical aperture is typically one order of magnitude lower |
| Scattered light is 10 -100 times weaker |
| High resolution and contrast | The resolution and contrast are much lower compared to those obtained with FF-OCT. |
In the future, OCT may replace frozen section as a non-invasive, intraoperative scanning method to assess adequate excisional margins.
III Postoperative Assessment
Moh’s microsurgery
Mohs micrographic surgery (MMS) was invented by Frederic E. Mohs in early 1930’s. It is considered as gold standard for the excision of locally invasive cutaneous malignancies in human dermatological surgery [24]. It is a unique horizontal sectioning technique, which enables 100% surgical margin assessment and provides the lowest recurrence rates. MMS uses a Mohs map with a schematic two-dimensional representation of the surgical site, combining measurement and charting of the tumour excision site.
Indications
Tumours in cosmetically sensitive areas
Tumours that have recurred after initial excision (e.g., eyelid periorbital area, nasolabial fold, nose-cheek angle, posterior cheek sulcus, pinna, ear canal, forehead, scalp, fingers, and genitalia).
To treat tumours with poorly defined clinical borders
Histologically aggressive, non melanoma skin cancer
Tumours with perineural invasion – SCC
Tumours arising in scarred or irradiated skin.
Procedure [24]: Excision of the tumour tissue has to be done at 45° angle to the surface in the shape of a bowl. This angled excisional method which is peculiar to MMS enables to flatten the tissue so that the entire depth and peripheral margins of the tumour can be sectioned in a horizontal plane. A two dimensional map should be drawn for reference.
Tissue handling in MMS can be done by two methods
Frozen technique
Formalin technique
Frozen Technique
After the excision is complete, the tissue is subdivided into sections small enough to fit on a glass slide. Smaller sections freeze more quickly than large specimens and are easier to manipulate into a horizontal plane. Maintaining the orientation of the tissues with respect to the Mohs map, sections have to numbered and cut edges to be dyed with different colours. Horizontal sections are taken with cryostat.
Formalin Technique
Tissue is oriented to the site map, fixed in formalin overnight, dyed and sectioned horizontally.
Histopathological assessment: Each section to be assessed for quality and location of neoplastic cells based on the mapped orientation. Microscopic findings and location of residual tumour cells are indicated and denoted on the Mohs map with red ink.
Hence, it was hypothesised that MMS could provide 100% margin assessment.
Ultrasonography
A quick and efficient method to confirm the surgical clearance of a resected fresh specimen is fine ultrasound imaging.
Procedure: After completion of the resection, the resected fresh specimen is immersed in the already prepared gelatine solution, maintaining its original shape and orientation with the help of some thread, slings, and refrigerated for about 20min for solidification of the gelatine. Ultrasound observation of the solidified gelatine-embedded specimen is performed from the superior surface. [Table/Fig-4] shows advantages and disadvantages of the role of ultrasonography in evaluating surgical margins.
Pros and cons of ultrasonography
| Advantage | Disadvantage |
|---|
| The ultrasound imaging of the fresh specimen when compared with the subsequently prepared histopathological sections shows clear images without distortion | Needs additional time to prepare gelatin solution |
Ultrasound imaging is used extensively in the assessment of the cervical lymphatics for regional metastasis. However, its role in evaluating the primary tumour has not been well investigated [25]. Ultrasonographic detection of close surgical margins has a sensitivity of 83% and a specificity of 63%. In tumours smaller than 5mm in thickness, CT and MRI could delineate the extent of the tumour with a density difference from normal tissue. High-quality ultrasonographic images can measure the tumour thickness within 1mm [26]. High-resolution ultrasonography is a valuable, noninvasive tool in assessing OSCC tumour margins and skin involvement and in determining the depth of lesions in the tongue [26]. Although, not a substitute for frozen-section assessment of pathologic margins in delineating surgical margins, ultrasonography acts as a promising tool for aiding surgeons in decision-making [27].
Molecular Assessment
Local recurrence occurs in up to half of patients with even microscopically negative surgical margins. Molecular studies indicate that there are two different mechanisms responsible in these cases. First, small clusters of residual tumour cells that are undetectable on routine histopathological examination (known as minimal residual cancer: MRC). A second cause of relapse is a remaining field of preneoplastic cells that is struck by additional genetic hits leading to invasive cancer [28].
The earliest stages of metastasis to the neck can be difficult to identify by light microscopy. It can be done using an assay based on the polymerase chain reaction (PCR) which is capable of detecting 1 mutant cancer cell among 10,000 normal cells.
P53, Ki67,K-ras & EGFR
A study conducted by Joseph AB et al., showed that SCC of the head and neck had a p53 mutation. (Okazaki Y et al.,) Epithelial dysplasia, with positive staining for p53 and Ki-67, has the potential for morphological changes, with possible recurrence of epithelial dysplasia or malignant transformation. No correlation was found between positivity of p53 or Ki-67 and the severity of dysplasia based on the WHO criteria. Genetic analysis of p53 and Ki-67 does not necessarily correlate with the histological degree of epithelial dysplasia, but rather is associated with early morphologic changes in the epithelium [29]. Detection of K-ras mutation in histologically negative surgical margins of pancreatic cancer correlates with less favourable clinical outcomes [30]. Overexpression of EGFR in tumor cells has been proved [31].
A study conducted by Carvalho A C D et al., [32] showed overexpression of PTHLH, EPCAM, MMP9 genes in positive margins. (parathyroid hormone-like hormone, epithelial cell adhesion molecule)
Gene Signature
Histologically normal margins may harbor underlying genetic changes, which increase the risk of recurrence. Genetic alterations identified in HNSCC included over-expression of eIF4E , TP53 and CDKN2A/P16 proteins.Other alterations included promoter hypermethylation of CDKN2A/P16 and TP53 mutations. In addition, promoter hypermethylation of CDKN2A, CCNAI and DCC was associated with decreased time to head and neck cancer recurrence. A gene signature can accurately predict which patients with OSCC are at a higher risk of disease recurrence [25].
Conclusion
The presence of tumour at the resection margin, and close to the resection margin should be considered separately with respect to prognostic significance. Intra-operative assessment of resection margins needs to emphasise involvement and proximity of tumour to the deep resection margin. The status of the surgical resection is an important predictor of outcome for both local recurrence and overall survival in oral cancer. As many techniques are available, depending upon the feasibility, a correct method has to be followed by a surgeon for complete clearance of the surgical margins to reduce the chances of recurrence.
[1]. Mehrotra R, Singh M, Gupta RK, Singh M, Kapoor AK, Trends of prevalence and pathological spectrum of Head and neck cancers in North IndiaIndian J Cancer 2005 42(2):89-93. [Google Scholar]
[2]. Reis PP, Waldron L, Perez-Ordonez B, Pintilie M, Galloni NN, Xuan Y, A gene signature in histologically normal surgical marginsBMC Cancer 2011 11:437 [Google Scholar]
[3]. Massano J, Regateiro FS, Januario G, Ferreira A, Oral squamous cell carcinoma: Review of prognostic and predictive factorsOral Surg Oral Med Oral Pathol Oral Radiol Endod 2006 102:67-76. [Google Scholar]
[4]. Kalavrezos N, Bhandari R, Current trends and future perspectives in the surgical management of oral cancerOral Oncology 2010 46(6):429-32. [Google Scholar]
[5]. Woolgar JA, Triantafyllou A, A histopathological appraisal of surgical margins in oral and oropharyngeal cancer resection specimensOral Oncology 2005 41(10):1034-43. [Google Scholar]
[6]. Kerawala CJ, The role of vital tissue staining in the marginal control of oral squamous cell carcinomaInt J Oral Maxillofac Surg 2000 29:32-5. [Google Scholar]
[7]. Missmann M, Jank S, Laimer K, Gassner R, A reason for the use of toluidine blue staining in the presurgical management of patients with oral squamous cell carcinomasOOOO E 2006 102:741-43. [Google Scholar]
[8]. Kurita H, Sakai H, Kamata T, Koike T, Kobayashi H, Kurashina K, Accuracy of intraoperative tissue staining in delineating deep surgical margins in oral carcinoma surgeryOral Oncol 2008 Oct 44(10):935-40. [Google Scholar]
[9]. Hansen DA, Spence AM, Carski T, Berger MS, Indocyanine green (ICG) staining and demarcation of tumor margins in a rat glioma modelSurgical neurology 1993 40(6):451-56. [Google Scholar]
[10]. Poh CF, Zhang L, Anderson DW, Durham JS, Williams PM, Priddy RW, Fluorescence Visualization Detection of Field Alterations in Tumor Margins of Oral Cancer PatientsClin Cancer Res 2006 12:6716-22. [Google Scholar]
[11]. Keller MD, Majumder SK, Kelley MC, Meszoely IM, Boulos FI, Olivares GM, Autofluorescence and diffuse reflectance spectroscopy and spectral imaging for breast surgical margin analysisLasers Surg Med 2010 42(1):15-23. [Google Scholar]
[12]. Chiou WY, Lin HY, Hsu FC, Lee MS, Ho HC, Su YC, Buccal mucosa carcinoma: surgical margin less than 3 mm, not 5 mm, predicts locoregional recurrenceRadiation Oncology 2010 5:79 [Google Scholar]
[13]. Nason RW, Binahmed A, Pathak KA, Abdoh AA, Sandor GKB, What is the adequate margin of surgical resection in oral cancer?OOOOE 2009 107(5):625-29. [Google Scholar]
[14]. Wong LS, McMohan J, Devine J, McLellan D, Thompson E, Farrow A, Influence of close resection margins on local recurrence and disease-specific survival in oral and oropharyngeal carcinomaBritish Journal of Oral and Maxillofacial Surgery 2012 50:102-08. [Google Scholar]
[15]. Novita G, Filassi JR, Ruiz CA, Ricci MD, Pincerato KM, de Oliveira Filho HR, Frozen Section Examination of the Margins for Resection of Squamous Cell Carcinoma of the Lower Lip 2003 61(8):890-94. [Google Scholar]
[16]. Olson TP, Harter J, Munoz A, Mahvi DM, Breslin T, Frozen section analysis for intraoperative margin assessment during breast-conserving surgery, results in low rates of re-excision and local recurrenceAnn Surg Oncol 2007 14(10):2953-60. [Google Scholar]
[17]. Klimberg VS, Westbrook KC, Korourian S, Use of touch preps for diagnosis and evaluation of surgical margins in breast cancerAnnals of surgical Oncology 1998 5(3):220-26. [Google Scholar]
[18]. Andrew JC, Jo AS, Peter RY, Kim R, Geisinger Intraoperative Evaluation of Lumpectomy Margins by Imprint Cytology with Histologic CorrelationArchives of Pathology & Laboratory Medicine 2002 126(7):846-48. [Google Scholar]
[19]. Yadav GS, Donoghue M, Tauro DP, Yadav A, Agarwal S, Intraoperative imprint evaluation of surgical margins in oral squamous cell carcinomaActa cytol 2013 57(1):75-83. [Google Scholar]
[20]. Upile T, Fisher C, Jerjes W, Maaytah EL, Singh S, Sudhoff H, Recent technological developments: in situ histopathological interrogation of surgical tissues and resection marginsHead & Face Medicine 2007 3:13 [Google Scholar]
[21]. Lauren LL, Peter MV, Richard WP, Schwarz R, Polydorides DA, Teng MS, High-Resolution Optical Imaging of Benign and MalignantMucosa in the Upper Aerodigestive Tract: An Atlas for Image-Guided SurgeryISRN Minimally Invasive Surgery 2012 [Google Scholar]
[22]. Hamdoon Z, Jerjes W, McKenzie G, Jay A, Hopper C, Assessment of tumour resection margins using optical coherence tomographyHead & Neck Oncology 2010 2(1):07 [Google Scholar]
[23]. Nguyen TF, Zysk AM, Chaney JE, Kotynek JG, Oliphant J, Bellafiore JF, Intraoperative Evaluation of Breast Tumor Margins with OpticalCoherence TomographyCancer Res 2009 69(22):8790-96. [Google Scholar]
[24]. Bernstein JA, Hodgin EC, Holloway HW, Hedlund CS, Storey ES, Hubert JD, Mohs micrographic surgery: a technique for total margin assessment in veterinary cutaneous oncologic surgeryVeterinary and Comparative Oncology 2006 4(3):151-60. [Google Scholar]
[25]. Kalavrezos Nicholas, Bhandari Rishi, Current trends and future perspectives in the surgical management of oral cancerOral Oncology 2010 :429-32. [Google Scholar]
[26]. Prabhakaran SS, Ramani P, Thiruvengadam C, Veeraiyan S, Premkumar P, Natesan A, Ultrasonography-guided assessment of surgical margins in oral carcinoma— a study of three casesCommun Oncol 2008 5:78-81. [Google Scholar]
[27]. Tominaga K, Yamamoto K, Khanal A, Morimoto Y, Tanaka T, Kodama M, Intraoperative surgical clearance confirmation of tongue carcinomas using ultrasoundDentomaxillofacial Radiology 2007 36:409-11. [Google Scholar]
[28]. Braakhuis JM, Bloemena E, Leemans RC, Brakenhoff HR, Molecular analysis of surgical margins in head and neck cancer: More than a marginal issueOral Oncology 2010 46(7):485-91. [Google Scholar]
[29]. de Carvalho AC, Kowalki LP, Campos AHJFM, Soares FA, Carvalho AL, Vettore AL, Clinical significance of molecular alterations in histologically negative surgical margins of head and neck cancer patientsOral Oncology 2012 48(3):240-48. [Google Scholar]
[30]. Okazaki Y, Sato K, Takada A, Morisaki S, Watanabe A, Ozawa Y, Evaluation of Epithelial Dysplasia at the Surgical Margins in Patients with Early Tongue Carcinoma —Immunohistochemical Study of Recurrence of Epithelial DysplasiaAsian J Oral Maxillofac Surg 2007 19:89-95. [Google Scholar]
[31]. Kim J, Reber H A, Dry S M, Elashoff D, Chen S L, Umetani N, Unfavourable prognosis associated with K-ras genemutation in pancreatic cancer surgical marginsGut 2006 55:1598-605. [Google Scholar]
[32]. Vosoughhosseini S, Lotfi M, Fakhrjou A, Aghbali A, Moradzadeh M, Sina M, Analysis of epidermal growth factor receptor in histopathologically tumor-free surgical margins in patients with oral squamous cell carcinomaAfrican Journal of Biotechnology 2012 11(2):516-20. [Google Scholar]