Purpose: To study multidrug-resistance in Uropathogenic E. Coli (UPEC) isolated from non-hospitalized patients.
Materials and Methods: Altogether, 250 bacterial samples were collected from non-hospitalized patients. Their identifications were done on basis of Gram-staining, colony morphology, biochemical testing and PCR. Susceptibility testing was performed by using standard protocols which were recommended by CLSI.
Statistical analysis: For comparisons, statistical analysis was performed by using software, Graphpad Prism 5.0
Results: In total, 32% (n = 80) of the isolates were identified as E. Coli strains and their susceptibility patterns for different antibiotics were determined. The data indicated least resistance against tazocin [(TZP) -1.25%], amikacin [(AK) -1.8%], tigecycline [(TGC)- 2.5%] and nitrofurantoin [(F) -3.75%]. For both minocycline (MH) and sulzone (SUL), resistance rate was 5%, for gentamicin (CN), it was 16.25%, while higher resistances were observed against cephalothine [(KF)- 70%], cefotaxime [(CTX) -58.5%], ceftazidime [(CAZ)- 57.5%], cefepime [(FEP) -55%], cefuroxime and cefixime [(CXM) (CFM)- 53.75 %]. Resistance against ciprofloxacin (CIP) was 57.5%, for norfloxacine (NOR), it was 52.5% and incase of sparfloxacin (SPX), it remained 55%. High percentage of the isolates were resistant to cotrimoxazole [(SXT) -86%] and Amoxicillin [AMX-CLA (AMC)- 76%]. No resistance against meropenem (MEM) was observed.
Conclusion: Highest level of drug-resistance was observed against trimethoprim-sulfamethoxazole (TMP-SMZ) among clinical isolates of uropathogenic E. Coli collected from non-hospitalized patients.
Antibiotic susceptibility, Beta-lactamase, Cotrimoxazole, E. Coli, Plasmids, UPEC
Introduction
Uropathogenic Escherichia coli (UPEC) is one of the major causes of urinary tract infections [1]. Several studies have reported increasing trends in resistance against trimethoprim-sulfamethoxazole (TMP -SMZ) [2,3] fluoroquinolones and other antibiotics, including ciprofloxacin [4,5]. To reduce the rate of morbidity, an early treatment of UTIs is mandatory, which relays on empirical therapies. However, to initiate an effective empirical treatment, several factors must be taken into consideration, including geographical location, age and sex of the patient, and local antimicrobial resistance profiles of the pathogens. In this study, we investigated prevalences and antimicrobial susceptibility patterns of UPEC in non-hospitalized patients.
Materials and Methods
This study was carried out from August 2012 to September 2013 in the Department of Microbiology of Quaid-i-Azam University, Islamabad, Pakistan. Study population consisted of patients of different age groups, those attended the Federal Government Services Hospital (polyclinic), Islamabad and visited other specialist clinicians in the periphery. Altogether, 250 mid stream urine samples were collected from non-hospitalized patients who had symptomatic UTIs. Samples were analyzed macroscopically and microscopically, both by wet mount and Gram-staining. A calibrated wire loop (0.001ml) was used to inoculate each sample on cystin lactose electrolytes deficient agar (CLED, Oxide, England) that was aerobically incubated overnight at 37°C. Colony counts of >105 CFU/ml were considered to be significant. Biochemical testing and PCR were performed for the precise identification of bacterial isolates. Bacterial DNA was extracted by using phenol-chloroform method [6]. DNA extraction was confirmed by directly visualizing on agarose gel (Sigma, Germany). For the confirmation of detection of E. Coli, a pair of primers from proximal and distal conserved flanking regions of 16s rRNA was used. PCR conditions were as Sectionfollows; 95oC for 1 minute, followed by 35 cycles of denaturation at 95oC for 45sec, annealing at 56oC for 45sec, extension at 72oC for one minute and a final extension at 72oC for 10 mins. The amplified products were observed on agrose gel. For DNA size estimation, a known marker of 100bp (Solis Biodyne) was used.
Antibiotic susceptibility was performed on Muller Hinton agar (Oxide, England) by Kirby Bauer disc diffusion method as per CLSI 2012 guidelines [7]. The antibiotics discs were obtained from Bioanalyse, Turkey. The antibiotic discs and concentrations (μg) which were used were as follows; ciprofloxacin (CIP;05), sparfloxacin (SPX;10), norfloxacine (NOR;10), gentamicin (CN;10), amikacin (AK;30), tigecycline (TGC;15), minocycline (MH;30), cotrimoxazole, trimethoprime-sulphamethoxazole (SXT;25), meropenem (MEM;10), nitrofurantoin (F;300), cepfime (FEP;30), ceftazidime (CAZ;30), cefotaxime (CTX;30), cefixime (CFM;05), cefuroxime (CXM;30), cephalothine (KF;30), sulzone: cefoperazone-sulbactum (SUL;105), aztreonam (ATM;30), tazocin: tazobactum-piperacillin (TZP;110) and augmentin: amoxicillin-clavulanic acid (AMC;30). The presence of (Extended Spectrum Beta-lactamases) ESBLs was confirmed by doing a phenotypic detection that was performed according to CLSI 2012 [7] guidelines. The discs which were used were those of amoxicillin-clavulanic acid, cefotaxime, ceftazidime (3rd generation) and manobactum (aztreonam). Furthermore, all the isolates were screened for the presence of plasmids.
Results
Altogether, 250 bacterial samples were collected and 32% of the isolates (n= 80) were confirmed to be E. coli strains. Antibiotic susceptibility was performed by using different classes of antibiotics, which included aminoglycosides, carbepenems, cephlosporins (1st, 2nd, 3rd and 4th generation), monobactams, nitrofurantoin, quinolones, sulfonamides, glycylcyclines, tetracycline and beta-lactamase inhibitors. As has been shown in [Table/Fig-1], least resistance was observed against tazocin, followed by amikacin and tigecycline. Out of these three antibiotics, only tazocin showed an intermediate level of resistance, that remained 7.5%. Amongst the antibiotics which were tested, significantly higher numbers of the isolates showed resistance to cotrimoxazole, in comparison to the resistance that was shown to sparfloxacin (χ²= 18.83; df= 1; p ≤ 0.0001), norflaxacin (χ²= 21.45; df= 1; p ≤ 0.0001) and ciproflaxacin (χ²= 16.36; df= 1; p ≤ 0.0001). However, no significant difference was seen with respect to resistance of the isolates to cotrimoxazole and amoxicillin AMX-CLA (χ²= 2.626; df= 1; p = 0.1052). Overall, higher resistance rates against ciprofloxacin, norfloxacin and sparfloxacin were observed, while these antibiotics showed a lower intermediate resistance [Table/Fig-1]. Observed resistance rates against cefotaxime, ceftazidime, cefepime and cefixime remained above 50%. Furthermore, for nitrofurantoin (3.75%) and both minocycline and sulzone, resistance rates were 5%, that was significantly lower in comparison to that shown against cotrimoxazole (χ²= 106.4; df= 1; p ≤ 0.0001). In total, 16.25% of the isolates showed resistance to gentamicin. No resistance to meropenem was observed throughout 2this study. Prevalence of MDR strains of UPEC was investigated and it appeared that 77.5% of all the screened isolates were resistant to three or more than three of the tested antibiotics [Table/Fig-2], that generated 20 different drug resistance patterns [Table/Fig-3]. Phenotypic testing identified 43.25% of the isolates as ESBL producers and 30% of these ESBL positive isolates were MDR, that generated at least six different combinations of antibiotics [Table/Fig-4]. Significantly higher percentage of ESBL producing isolates showed resistance towards cotrimoxazole in comparison to quinolones (χ²= 7.793; df= 1; p ≤ 0.0052) In total, 88% of the UPEC isolates were plasmid positive [Table/Fig-1].
Discussion
Urinary tract infections (UTIs) are one of the most common infections seen worldwide [8]. Uropathogenic E. coli (UPEC) alone account for 70-90% of the UTI infections[9,10] and their susceptibility patterns against different antibiotics vary in different geographical regions, eventually leading to empirical therapy which is based on the local susceptibility profiles. Being done with the major objective of evaluating uropathogenic E. coli strains and their antibiotic resistances, this study highlighted that 86% of the tested UPEC isolates were resistant to trimethoprime sulfamethoxazole, which was significantly higher. Importantly, according to WHO recommendations, this antibiotic has been suggested as a first choice for UTI treatments [11]. Furthermore, we found that up to 76.3% of the isolates showed resistance towards co-amoxiclav and that 42% isolates showed resistance to fluoroquinolones [12-14]. Fluoroquinolones are considered as first choice for the treatment of UTIs in men, mainly because it has advantages over co-amoxiclav, which are related to its pharmacokinetic properties [15,16]. However, observed higher percentages of resistances against both drugs indicated that they could render their efficacies as therapeutic agents, particularly in Indian sub-continent. The percentages of resistances for both drugs appeared to be above the threshold level [10]. As an alternative choice, nitrofurantoin could be considered as a drug of choice, given the low level of resistance found against this antibiotic [17]. However, there is no data on the effectiveness of this drug in the treatment of male patients and pharmacokinetic properties of this antibiotic are not better than those of fluoroquinolones [16]. Prevalence of MDR strains of UPEC was investigated in this study and it appeared that 77.5% of all the screened isolates were resistant to three or more than three of the tested antibiotics. For MDR strains of UPEC, similar trends were observed in Iran (77%), whereas in India, they were 92%, in Slovenia, they were 42% and in USA, MDR rates were 7.1% [18]. No resistance against meropenem [12,13,19,20] and least resistance against tazocin 1.25% were observed in this study. For the treatment of MDR strains of UPEC, these antibiotics may be considered as an alternative choice; however, prior to the initiation of treatment, patient history should be taken into consideration. Higher rates of prevalence of MDR strains which are seen in some countries, including Pakistan, undermine options available for empirical therapy. Generally, cephalosporins are considered to be very effective against Gram-negative bacterial infections. Observed resistance rate for cephalosporins was 70% for the first generation drugs and the rates remained between 53.7 to 58.5% for second and third generation drugs respectively. Similarly, fourth generation cephalosporins appeared to be no exception as well, because 55% of the isolates showed resistance to cefepime. Similar findings had been previously observed in south east Asian region [12,13,19]. Reported resistance rate against these drugs was comparatively lower in Iran (19.6%)[20] and in Bangladesh, it was 32% [21]. Based on phenotypic testing, we found that 43.25% of the isolates were ESBL producers. In this context, role of plasmids in dissemination of resistance against multiple antibiotics has been widely acknowledged [22]. In case of extended-spectrum β-lactamase (ESBL) producing Escherichia coli, ESBL enzymes have been reported to be plasmid encoded [23]. We found that 88% of UPEC isolates carried plasmids. Exact roles of these plasmids in drug resistance has not been determined in this study. Obviously, higher percentages of ESBL producers and multidrug resistant strains of UPEC put further constraints on necessary therapeutic measures. For countries which have higher percentages of drug resistance, it is important to integrate antibiotic susceptibility testing in routine diagnostic practices.
Shown are the numbers and percentages of drug resistant ESBL and non-ESBL Uropathogenic E. coli
| Drug resistance among UPEC | Drug resistance among ESBL +ve UPEC |
| Antibiotics | Resistant N (%) | Intermediate N (%) | Resistant N (%) | Intermediate N (%) | Plasmid +ve N (%) |
| Amikacin | 2 (2.5) | 0 (0) | 0 (0) | 0 (0) | 1(50) |
| Gentamicin | 13 (16.25) | 3 (3.75) | 4(9.3) | 3(6.9) | 6(46.15) |
| Meropenem | 0 (0) | 0 (0) | | 0 (0) | 0 (0) |
| Cephalothine | 56(70) | 9 (11.25) | - | - | 55(98.21) |
| Cefuroxime | 43(53.75) | 2 (2.5) | - | - | 42(97.67) |
| Ceftazidime | 45(56.70) | 1(2.5) | - | - | 44(93.33) |
| Cefotaxime | 47(58) | 3(3.7) | - | - | 46(98) |
| Cefixime | 47(58.75) | 0 (0) | - | - | 46(98) |
| Cefepime | 44(55%) | 0 (0) | - | - | 43(97.7) |
| Aztreonam | 45(56.25) | 0(0) | - | - | 44(97.7) |
| Nitrofurantoin | 3(3.75) | 0 (0) | 01(2.3) | 1(2.3) | 0(0) |
| Co-amoxiclav | 61(76.30) | 3 (3.7) | - | - | 60(98.3) |
| Ciprofloxacin | 46 (57.5) | 1(1.25) | 22(51.1) | 0(0) | 44(95.6) |
| Norfloxacin | 42(52.5) | 3 (3.75) | 22(51.1) | 1(2.3) | 40(95.23) |
| Sparfloxacin | 44(55) | 3 (3.75) | 22(51.1) | 3(6.9) | 42(95.4) |
| Sulfamethoxazol | 69(86. 25) | 9(11.25) | 35(81.3) | 0(0) | 67(97.1) |
| Tigecycline | 2 (2.5) | 0 (0) | 0 (0) | 1(2.3) | 0(50) |
| Minocycline | 4(5) | 9 (11.25) | 2(4.6) | 5(11.6) | 1(25) |
| Tazocin | 1(1.25) | 6 (7.5) | - | - | 0(0) |
| Sulzone | 4 (5) | 4 (5) | - | - | 1(25) |
| E (20%) | 10.7 | 3.68 | 3.68 | 3.68 | 3.68 |
| E (20%) | 10.7 | 3.68 | 3.68 | 3.68 | 3.68 |
The numbers of MDR-UPEC resistant to at least three or more than three drugs are depicted in graph. Combinations of antibiotic represent as C1-C20 can be seen separately in [Table/Fig-3]
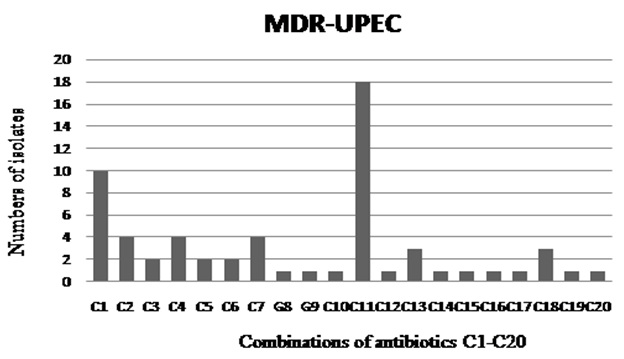
Given are the combinations of antibiotics based on the resistant profile of MDR-UPEC, numbers of isolates resistant to each group from C1- C20 are separately shown in [Table/Fig-2]
| *C1 | C2 | C3 | C4 | C5 | C6 | C7 | C8 | C9 | C10 | C11 | C12 | C13 | C14 | C15 | C16 | C17 | C18 | C19 | C20 |
| AMC | AMC | CIP | AMC | AMC | AMC | AMC | AMC | AMC | AMC | AMC | SXT | AMC | AMC | CIP | CN | CIP | AMC | AMC | AMC |
| ATM | SXT | SPX | ATM | ATM | ATM | ATM | ATM | CN | ATM | ATM | KF | ATM | ATM | SPX | CIP | SPX | CIP | SXT | |
| CAZ | KF | NOR | CAZ | CAZ | CAZ | CAZ | CAZ | SXT | CAZ | CAZ | MH | CAZ | CAZ | SXT | SPX | SXT | SPX | FEP | CFM |
| CFM | - | KF | CFM | CFM | CFM | CFM | CFM | KF | CFM | CFM | - | CFM | CFM | - | SXT | KF | NOR | - | CXM |
| CXM | - | CN | CXM | CXM | CXM | CXM | CXM | - | CXM | CXM | - | CXM | CXM | - | KF | - | SXT | - | FEP |
| CN | - | - | CTX | CN | CTX | CN | CN | - | CN | CTX | - | CTX | CTX | - | NOR | - | KF | - | CTX |
| CTX | - | - | CIP | CTX | SXT | CTX | CTX | - | CTX | CIP | - | CIP | CIP | - | - | - | - | - | CIP |
| CIP | - | - | SPX | CIP | KF | CIP | CIP | - | SXT | SPX | - | SPX | SPX | - | - | - | - | - | SPX |
| SPX | - | - | NOR | SPX | FEP | SPZ | SPZ | - | KF | NOR | - | NOR | NOR | - | - | - | - | - | MH |
| NOR | - | - | MH | NOR | - | NOR | NOR | - | FEP | SXT | - | SXT | SXT | - | - | - | - | - | SXT |
| SXT | - | - | TZP | MH | - | MH | TZP | - | NOR | KF | - | KF | KF | - | - | - | - | - | KF |
| KF | - | - | SXT | TZP | - | SXT | SUL | - | - | FEP | - | FEP | FEP | - | - | - | - | - | NOR |
| FEP | - | - | AK | SXT | - | KF | KF | - | - | - | - | F | F | - | - | - | - | - | - |
| - | - | KF | AK | - | NOR | FEP | - | - | - | - | - | CN | - | - | - | - | - | - |
| - | - | FEP | KF | FEP | - | - | - | - | - | - | - | TZP | - | - | - | - | - | - |
| - | - | | FEP | - | - | | - | - | - | - | - | SUL | - | - | - | - | - | - |
Antibiotic resistance profile of ESBL isolates those are MDR-UPEC
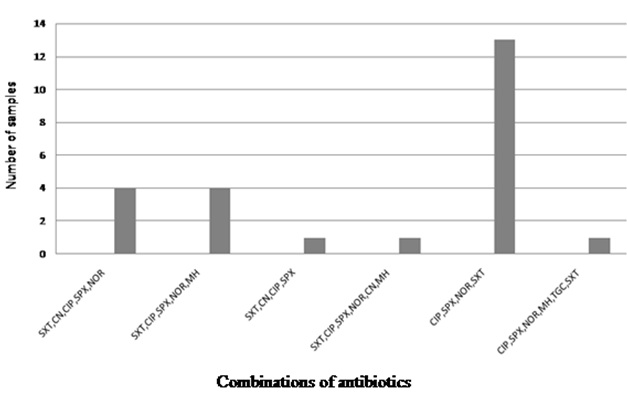
Conclusion
Higher percentages of UPEC which are isolated from non-hospitalized patients are being reported, a majority of these being ESBL producers and MDR. This report was consistent with findings of other studies which were previously conducted in this region, which had confirmed that higher percentages of the UPEC isolates were resistant to trimethoprime sulfamethoxazole and fluoroquinolones. Nitrfurontine can be considered to be effective against them. This study was conducted in one region and given its small sample size, it may not reflect the overall situation which is prevalent throughout the country or in a wider region. Thus, conducting national surveillance programs for MDR-UPEC in this region, should be given priority.
Acknowledgement
This study was supported by Higher Education Commission Pakistan IPFP grant NO-3782. The authors would like to thank Dr. Rizwan Uppal and Dr. Khursheed Ahmed from IDC-Islamabad for providing technical support.
[1]. A Ronald, The etiology of urinary tract infection: traditional and emerging pathogens.Dis Mon. 2003 49:71-82. [Google Scholar]
[2]. DF Sahm, C Thornsberry, DC Mayfield, ME Jones, JA Karlowsky, Multidrug-resistant urinary tract isolates of Escherichia coli: prevalence and patient demographics in the United States in 2000.Antimicrob Agents Chemother. 2001 45:1402-06. [Google Scholar]
[3]. S Nys, T Van Merode, AI Bartelds, EE Stobberingha, Antibiotic treatment and resistance of unselected uropathogens in the elderly.Int J Antimicrob Agents. 2006 27:236-41. [Google Scholar]
[4]. JA Karlowsky, DJ Hoban, MR Decorby, NM Laing, GG Zhanel, Fluoroquinolone-resistant urinary isolates of Escherichia coli from outpatients are frequently multidrug resistant: results from the North American Urinary Tract Infection Collaborative Alliance-Quinolone Resistance study.Antimicrob Agents Chemother. 2006 50:2251-54. [Google Scholar]
[5]. CH Park, A Robicsek, GA Jacoby, DF Sahm, DC Hooper, Prevalence in the United States of aac(6′)-Ib-cr encoding a ciprofloxacin-modifying enzyme.Antimicrob Agents Chemother. 2006 50:3953-55. [Google Scholar]
[6]. HR Cheng, N Jiang, Extremely rapid extraction of DNA from bacteria and yeasts.Biotechnol Lett. 2006 28:55-59. [Google Scholar]
[7]. Clinical and Laboratory Standards Institute (CLSI): Performance standards for antimicrobial susceptibility testing, 22th informational supplement. CLSI document M100–S22Clin Pediatr 2012 :32-3. [Google Scholar]
[8]. AR Ronald, LE Nicolle, E Stamm, J Krieger, J Warren, A Schaeffer, Urinary tract infection in adults: Research priorities and strategies.Int J Antimicrob Agents. 2001 17:343-48. [Google Scholar]
[9]. P Ulleryd, Febrile urinary tract infection in men.Int J Antimicrob Agents 2003 22:89-93. [Google Scholar]
[10]. K Gupta, TM Hooton, WE Stamm, Increasing antimicrobial resistance and the management of uncomplicated community-acquired urinary tract infections.Ann Intern Med. 2001 135:41-50. [Google Scholar]
[11]. O Wolff, C Maclennan, Evidence behind the WHO guidelines hospital care for children: What is the appropriate empiric antibiotic therapy in uncomplicated urinary tract infection in children in developing countries?J Trop Ped. 2007 53:150-52. [Google Scholar]
[12]. R Tanvir, R Hafeez, S Hasnain, Prevalence of Multiple Drug Resistant E. Coli in patients of Urinary tract infection registering at a Diagnostic Laboratory in Lahore.Pakistan J Zool. 2012 44:707-12. [Google Scholar]
[13]. A Sotto, DM Crinne, B Parcale, F Peray, A Gouby, D Jacques, Risk factors for antibiotics resistant E. Coli isolated from hospitalized patients with U.T.I: A prospective study.J Clin Microbiol. 2001 39:438-44. [Google Scholar]
[14]. S Bashir, Y Sarwar, A Ali, M Mohsin, MA Saeed, A Tariq, Multiple drug resistance patterns in various phylogenetic groups of uropathogenic E. Coli.Brazilian Journal of Microbiology. 2011 42:1278-83. [Google Scholar]
[15]. FM Wagenlehner, W Weidner, KG Naber, Therapy for prostatitis, with emphasis on bacterial prostatitis.Expert Opin Pharmacother. 2007 8:1667-74. [Google Scholar]
[16]. K Charalabopoulos, G Karachalios, D Baltogiannis, A Charalabopoulos, X Giannakopoulos, N Sofikitis, Penetration of antimicrobial agents into the prostate. Chemotherapy. 2003 49:269-79. [Google Scholar]
[17]. DR Guay, An update on the role of nitrofurantoin in the management of urinary tract infections.Drugs. 2001 61:353-64. [Google Scholar]
[18]. JA Linder, ES Huang, MA Steinman, R Gonzales, RS Stafford, Fluoroquinolone prescribing in the United States: 1995 to 2002.Am J Med. 2005 118:259-68. [Google Scholar]
[19]. M Akram, M Shahid, AU Khan, Etiology and antibiotic resistance patterns of community-acquired urinary tract infections in J N M C Hospital Aligarh, India, Annals of Clinical Microbiology and Antimicrobials. 2007 :6-4. [Google Scholar]
[20]. S Farshad, R Ranjbar, A Japoni, M Hosseini, M Anvarinejad, R Mohammadzadegan, Microbial Susceptibility, Virulance Factor, and Plasmid profiles of Uropathogenic E. Coli Strains Isolated from Children in Jahrom.Iran Archives of Iranian Medicine. 2012 15:5 [Google Scholar]
[21]. M Marhova, S Kostadinova, S Stoitsova, Antimicrobial resistance profiles of urinary Escherichia coli isolates. Biotechnol and Biotechnol. 2009 Special edition/on-line [Google Scholar]
[22]. K Bush, Bench-to-bedside review: the role of beta-lactamases in antibioticresistant Gram-negative infections.Crit Care. 2010 14:224 [Google Scholar]
[23]. DL Paterson, RA Bonomo, Extended-spectrum beta-lactamases: a clinical update.Clin Microbiol Rev. 2005 18:657-86. [Google Scholar]