Background: The characteristic cytologic features of the common salivary gland lesions have been well-delineated in literature. However, there also exist cytologic pitfalls and overlapping features that make an accurate diagnosis difficult in few cases. The present study was designed to compare the cytologic findings of salivary gland lesions with the histologic diagnoses, in order to assess the sensitivity, specificity and diagnostic accuracy of FNAC, with an emphasis on discordant cases.
Materials and Methods: Patients with suspected salivary gland enlargements, who were referred for FNAC, were included in this study, which was done over a 3 year period in a medical college hospital. FNAC was performed by using the standard procedure. Smears were stained by using Papanicolaou’s and MGG stains. Cytologic diagnosis was compared with histopathologic diagnosis wherever it was available.
Results: Eighty eight patients with salivary gland swellings were included in the study. The ages of the patients ranged from 15 to 82 years, with the M:F ratio being 1.6:1. Out of 88 cases, 68 had swellings in parotid gland, 19 had them in submandibular gland and one had them in hard palate. Pleomorphic adenoma was the commonest neoplasm which was seen in our study. Mucoepidermoid carcinoma (MEC) was the only malignant lesion seen in our study. One each of Warthin’s tumour (WT) and MEC were overdiagnosed and underdiagnosed respectively, the reason being squamous metaplasia in WT and subtle nature of malignant cells in low-grade MEC.
Conclusion: WT and MEC can pose problems in cytologicdiagnosis. Sampling errors and interpretational errors can lead todiscordant diagnoses.
Introduction
Salivary gland swellings can result from tumours, an inflammatory process or cysts. It can sometimes be difficult to establish as to whether pathology arises from the salivary gland itself or from adjacent structures such as lymph nodes, soft tissues or skin. Fine Needle Aspiration Cytology (FNAC) is a widely used, safe and relatively nontraumatic procedure that can quickly provide importantinformation. The main goal of doing FNAC of salivary gland lesionsis to assist clinicians in the management of patients who present with mass lesions. The characteristic cytologic features of common salivary gland lesions have been well-delineated in literature [1]. However, there also exist cytologic pitfalls and overlapping features that make an accurate diagnosis difficult in few cases. This has led to a wide-range of sensitivities (62-97.6%) and specificities (94.3- 100%) of cytologic diagnosis [2-5].
The present study was designed to compare the cytologic findings of salivary gland lesions with their histologic diagnoses, in order to assess the sensitivity, specificity and diagnostic accuracy of FNAC, with an emphasis on discordant cases.
Materials and Methods
This study was performed on patients with suspected salivary gland swellings, who were referred for FNAC to the Department of Pathology of a medical college and hospital. This was done over a period of three years, on patients of either sex or any age, after obtaining written consents from them. Relevant clinical details were elicited in all the cases and findings of local examinations done were noted. All the patients underwent FNAC with use of a 23 G needle, with suction being provided by a 10 ml syringe. The characters of aspirates were noted, routine smears were prepared and they were stained with May-Grünwald’s-Giemsa and Papanicoloau’s stains.
The cytologic interpretation was done as it has been described in standard textbooks of cytology. Histologic correlations were done wherever it was possible. A histologic diagnosis was considered as the gold standard for assessment of sensitivity and specificity of FNAC. A cyto-histologic correlation was done and appropriate statistical tests were applied.
Results
Eighty eight patients with salivary gland swellings were included in the study. The ages of the patients ranged from 15 to 82 years, with M:F ratio being 1.6:1. Out of 88 cases 68 had swellings in parotid gland, 19 had them in submandibular gland and one had them in hard palate. Cytological diagnoses offered for different cases have been listed in [Table/Fig-1]. Cytomorphologies of pleomorphic adenomas, myoepitheliomas , Warthin’s tumours and mucoepidermoid tumours have been shown in [Table/Fig-2,3,4,5] . Histopathologic correlations were available for 31 cases.
Discussion
FNAC is a technique which is commonly used for the evaluation of both neoplastic and non neoplastic lesions which occur salivary gland [5-7],. The parotid gland was the commonly studied gland, followed by submandibular gland, a finding which has been well described in the literature [1-9].
Most common lesion was pleomorphic adenoma (45%), with a majority of such tumours showing involvement of parotid gland, with 100% diagnostic accuracy. In the parotid gland, pleomorphic adenomas account for more than 70% of all the tumours, but they rarely occur in the sublingual gland [10]. Cytologically, the smears show 3 main components: extracellular matrix, myoepithelial and ductal cells which are present in various proportions, and stroma [10]. The diagnosis of a pleomorphic adenoma is usually made obvious after the identification of the 3 components which have just been described. However, the considerable variation in the proportions of the constituent elements is a challenge, because of the resultant long list of differential diagnoses. Stroma-deficient or cellular cases may be difficult to recognize as pleomorphic adenomas and they may be confused with other tumours of the salivary gland. The differential diagnoses include low-grade carcinomas, monomorphic adenomas and metastases, and theplasmacytoid appearance of the myoepithelial cells may be mistaken for malignant lymphomas or plasma cell proliferations [10-12].
Out of five cytologically diagnosed Warthin’s tumours (WT), four were available for histopathological studies. Two were WT, one was low grade MEC and one showed chronic siadenitis with extensive squamous metaplasia. WT is a benign tumour which is commonly encountered in salivary gland FNAC specimens. Histologic examination of WT reveals the characteristic cystic spaces which are lined by a bilayer of oncocytic cells and abundant lymphocytes in the subepithelial stroma. The aspirated material appears chocolate brown. The cellularity of the smears is variable and it may be quite hypocellular, owing to fluid dilution. The three main components that characterize the FNAC cytology of WT are oncocytes, lymphocytes, and the fluid background [1-10]. In addition to the three main components, other elements that can be encountered in FNA smears of WT include, albeit rarely, metaplastic squamous and sebaceous gland cells. The differential diagnoses for WT are wide [2,4]. In most of the cases (>80%), the presence and combination of the three main cytologic elements can help in establishing the diagnosis without difficulty. However, as is well known, oncocytes, lymphocytes, and a fluid background are not pathognomonic of WT, as they are encountered in several other conditions also. Cysts (lymphoepithelial cysts) of the salivary glands and chronic inflammatory and obstructive duct lesions can accumulate fluid, show oncocytic metaplasia, contain numerous lymphocytes and can be easily confused with WT [10]. The 2intermediate squamous cells of MEC, the uncommon oncocytic metaplasia of pleomorphic adenomas, and metaplastic cells of squamous cell carcinomas, may all be confused with WT [13]. It is important to always reaspirate any residual mass after initial drainage of fluid from WT cases or any other cystic lesions, to obtain a more representative material which may provide clues to the diagnosis [10].
Diagnostic accuracy for myoepithelioma in the present study was 100%. Myoepitheliomas are uncommon, benign neoplasms that occur in the parotid and submandibular glands and the palate. Pleomorphic adenomas and malignant myoepitheliomas are the chief differential considerations for myoepitheliomas [10].Identification of plasmacytoid myoepithelial cells which are intimately mixed with metachromatic stroma, favours a diagnosis of pleomorphic adenomas, although stroma-poor cases may be difficult to distinguish from myoepitheliomas. The cytologic features of the extremely rare malignant myoepitheliomas have not been well reported, but the presence of significant nuclear atypia and mitosis may suggest such a diagnosis.
In our study,10 cystic lesions were seen on cytology. Four were available for histopathologic evaluations. One MEC, two Warthin’s tumours and cystadenomas were encountered. This may have occurred because of a sampling error, where the needle might have hit only the cystic area. Therefore, it is advisable to suggest excisions for cystic lesions. Cystic lesions of the salivary glands can either be non-neoplastic or malignant [4]. Examples of non neoplastic cysts are lymphoepithelial cysts, retention cysts and mucoceles. WT is the commonest cystic neoplasm, but pleomorphic adenoma, mucoepidermoid tumour and acenic cell arcinoma can also be cystic.
Non neoplastic lesions constituted 19% of all salivary gland lesions seen in our study, which was similar to findings of other studies [5,7]. One case of chronic sialadenitis with squamous metaplasia was labelled as a Warthin’s tumour, based on cytological findings. Metaplastic squamous cells and lymphocytes of sialadenitis were interpreted as WT, based on cytological findings. Nonneoplastic lesions such as chronic sialadenitis are frequently encountered as mass lesions. Long standing chronic inflammations and duct obstructions may lead to metaplastic changes in the ductal epithelium, which include squamous, mucous and oncocytic metaplasia, which may be mistaken for a variety of benign and malignant neoplasms [10].
MEC was the only malignant lesion seen in our study. Out of four cases, three were available for histopathological studies. A case of a 15-year-old boy’s parotid aspirate was labelled as a WT on cytology, due to the cystic lesion, lymphocytes and squamoid cells which were present in it. Histopathological studies showed that it was a low grade mucoepidermoid carcinoma. Squamous cells and mucinous cells were interpreted as oncocytic cells and cyst macrophages respectively. Underdiagnoses of low grade MECs are a common problem, because of bland cytologic features and foamy cells which resemble histiocytes [10,14]. WT with extensive squamous metaplasia was labelled cytologically as a MEC in an adult. On reviewing, the smears showed numerous lymphocytes and metaplastic squamous cells. There were no intermediate type of squamoid cells. The differential diagnosis of low grade MECs includes WT, benign salivary gland cysts, branchial cleft cysts, sialolithiasis or chronic sialdenitis, with cystic dilatation and PA with excess mucoid stroma [1,10,14]. To differentiate Warthin’s tumours from lowgrade MECs, one should look for the presence of numerous oncocytes, especially in cohesive clusters, a dirty cystic background and lymphocytes. Presence of oncocytes and lymphocytes in large numbers is not usually seen in MECs [10]. Obstructive duct lesions with squamous metaplasia can mimic a mixed pattern of low grade MECs [4,10]. MECs can occur at any age, they can involve both the major and minor salivary glands, and they are the most common malignant salivary gland tumours which are seen in children.
The rates of false negative diagnoses made on cytology, which have been reported in the literature, range from 0 to 37% [5,15,16]. The false negative rate in our study was 25%. This had occurred because one case of MEC was misdiagnosed as WT. The false positive rate has been reported to be low ( 0-10%) [2,15,16]. In our series, the false positive rate was 4.8 % and this was due to one case of Warthin’s tumour, with extensive squamous metaplsia being diagnosed as MEC on FNAC. The sensitivity and specificity were 80% and 95% respectively. These results suggested that FNAC could serve as a good preoperative diagnostic tool for salivary gland lesions. A preoperative malignant diagnosis allows the surgeons to plan the treatment, while a benign diagnosis relieves the patient from anxiety and surgical procedures.
Cytological diagnosis in 88 cases of salivary gland aspirates
| Cytological diagnosis | Number | Histology available |
| Chronic Sialdenitis(CS) | 17 | 05 |
| Sialadenosis | 08 | - |
| Cystic lesion | 10 | 04 |
| Intraparotid lymph node | 02 | - |
| Pleomorphic adenoma(PA) | 40 | 14 |
| Warthin’s tumor(WT) | 05 | 04 |
| Myoepithelial tumor (MT) | 02 | 02 |
| Mucoepidermoid carcinoma (MEC) | 04 | 03 |
| Total | 88 | 32 |
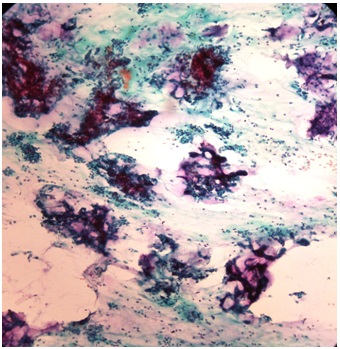
Smears from pleomorphic adenoma showing epithelial cells and chondromyxoid stroma (PAP 10X)
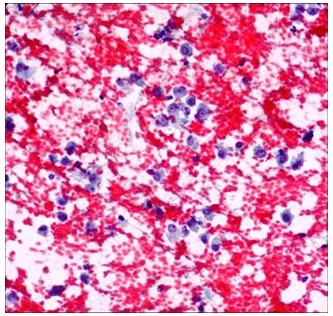
Smears from myoepithelioma showing plasmacytoid cells (PAP10X)
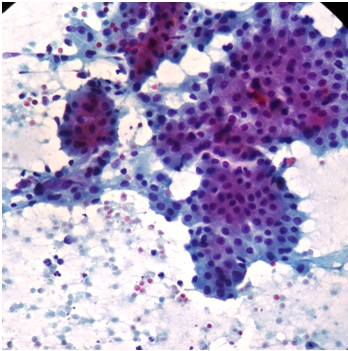
Smears from warhins tumor showing oncocytic cells and lymphocytes(PAP 20X)
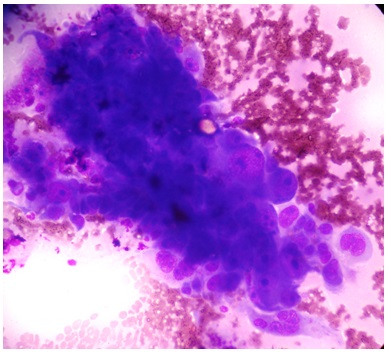
smears from mucoepidermoid carcinoma showing malignant squamoid cells (MGG)
Recommendations
A multidirectional aspiration is preferred, to avoid a selective sampling. Adequate knowledge of histologic heterogeneity of salivary gland tumours will help in making an appropriate diagnosis.
Limitations
Due to the pitfalls in cytologic diagnosis of certain salivary gland tumours, tissue biopsies may be necessary for doing histological examinations.
Conclusion
False negative results have been obtained mainly due to errors made in underdiagnosis of low grade tumours pertaining to their bland cytologic features and the difficult interpretation of hypocellular cystic lesions. False positive diagnoses emanate from reactive changes which are associated with inflammatory reactions.
[1]. G Kocjan, KA Shah, Salivary glands. In :Gray W, Kocjan G, editors. Diagnostic Cytopathology.Churchill Living stone 2010 3rd EditionEdinburgh:231-52. [Google Scholar]
[2]. G Kocjan, M Nayagam, M Harris, Fine needle aspiration cytology of salivary gland lesions: advantages and pitfalls.Cytopathology. 1990 1:269-75. [Google Scholar]
[3]. SR Orell, Diagnostic difficulties in the interpretation of fine needle aspirates of salivary gland lesions: the problem revisited.Cytopathology. 1995 6:285-300. [Google Scholar]
[4]. S Chakrabarthy, M Bera, PK Battacharya, D Chakra, AK Manna, S Pathak, Study of salivary gland lesions with fine needle aspiration cytology and histopathology along with immunohistochemistry.J Indian Med Assoc. 2010 108:833-36. [Google Scholar]
[5]. A Ashraf, AS Shaikh, F Kamal, R Sarfraz, MH Bukhari, Diagnostic reliability of FNAC for salivary gland swellings: A comparative study.Diagn Cytopathol. 2010 38:499-504. [Google Scholar]
[6]. A Singh, A Haritwal, BM Murali, Correlation between cytology and histopathology of the salivary gland.AMJ. 2011 2:66-71. [Google Scholar]
[7]. R Jain, R Gupta, M Kudesia, S Singh, Fine needle aspiration cytology in diagnosis of salivary gland lesions: A study with histologic comparison. Cyto Journal. 2013 10:5DOI: 10.4103/1742-6413.109547 [Google Scholar]
[8]. GC Fernandes, AA Pandit, Diagnosis of salivary gland tumors by FNAC.Bombay Hospital Journal. 2000 42:108-11. [Google Scholar]
[9]. Aan Noor ul, Tanwani Ashok Kumar, Pitfalls in Salivary Gland Fine-Needle Aspiration Cytology.International Journal of Pathology. 2009 7:61-5. [Google Scholar]
[10]. P Mukunyadzi, Review of Fine-Needle Aspiration Cytology of Salivary Gland Neoplasms, With Emphasis on Differential Diagnosis. Am J Clin Pathol. 2002 118:S100-S115S.(Suppl 1) [Google Scholar]
[11]. MW Stanley, T Lowhagen, ucin production by pleomorphic adenomas of the parotid gland: a cytologic spectrum.Diagn Cytopathol. 1990 6:49-52100. [Google Scholar]
[12]. U Handa, N Dhingra, R Chopra, H Mohan, Pleomorphic Adenoma: Cytologic variations and potential pitfalls. Dign Cytopathol.37:12-15. [Google Scholar]
[13]. J Klijanienko, P Vielh, Fine-needle sampling of salivary glandlesions, II: cytology and istology correlation of 71 cases of Warthin’s tumor (adenolymphoma).Diagn Cytopathol. 1997 16:221-25. [Google Scholar]
[14]. MW Stanley, SHead and neck cytology. In: Silverberg SG, ed.Principles and Practice of Surgical Pathology and Cytopathology. 1997 23rd EditionNew YorkChurchill Livingstone:995-1037. [Google Scholar]
[15]. Y Daneshbod, K Daneshbod, B Khademi, Diagnostic difficulties in the interpretation of fine needle aspirate samples in salivary lesions: Diagnostic pitfalls revisited.Acta Cytol. 2009 53:53-70. [Google Scholar]
[16]. LJ Layfield, E Gopez, S Hirschowitz, Cost efficiency analysis for fine-needle aspiration in the workup of parotid and submandibular gland nodules.Diagn Cytopathol. 2006 34:734-38. [Google Scholar]