Acne vulgaris is a common dermatological disorder with a prevalence reported to reach upto 85% of population across various regions [1]. It has a significant impact on the quality of life [2,3], psychosocial development as well as self-esteem of the patients [4]. It affects areas of the skin having the highest population of sebaceous follicles, most common being the face, the upper part of chest, and the back [5].
Until recently follicular epidermal hyperproliferation with subsequent plugging of the follicles (comedones) was considered to be the earliest event in the development of acne and closed comedones were regarded as the precursors of inflammatory lesions [6,7].Current evidence has shown that inflammatory events can precede microcomedone formation and that the development of follicular duct plugs is also influenced, to some degree, by inflammation caused by Propionibacterium acnes [6,8,9]. P. acnes is an anaerobic organism present in the sebaceous glands which contributes to the pathophysiology of acne in several ways. It stimulates inflammation by producing pro-inflammatory mediators through toll like receptor-2 (TLR-2) and activation of the innate immune system [8–10].
A topical nano-emulsion gel preparation containing clindamycin (as phosphate) 1% w/w as the active ingredient has been formulated by a unique nano-emulsion technology. Nano-emulsions have a much higher surface area than regular macro-emulsions and thus have good penetration into the pilo-sebaceous glands, providing better efficacy. Further, the aqueous-based gel vehicle of the preparation has moisturising properties which can improve local tolerability. The present study was undertaken to assess the efficacy and safety of therapy with this nano-emulsion gel formulation of Clindamycin 1% in comparison with its conventional gel formulation in patients suffering from acne vulgaris of the face.
Materials and Methods
This prospective, randomized, open label, active controlled, multicentric, phase IV clinical study was carried out at seven study centres across India from October 2010 to May 2011. The study was reviewed and approved by an Independent Ethics Committee (IEC) for all the participating study centres before enrolment of the first patient. The study was conducted in compliance with the GCP Guidelines issued by International Conference on Harmonisation (ICH-GCP) and the ethical principles of Declaration of Helsinki. All the participating patients provided written informed consent before enrolment into the study.
Patients
Male and female patients of at least 12 years age with an established diagnosis of acne vulgaris of the face who were likely to be available for all follow-up visits were enrolled in the study. Female patients were required not to be pregnant or lactating at the time of enrolment and not planning pregnancy during the study period.
Patients with a history of regional enteritis, ulcerative colitis or antibiotic-associated colitis; significant cardiovascular, hepatic, renal or any other systemic illnesses were excluded from the study. Patients with hypersensitivity to preparations containing clindamycin, lincomycin, or any other related class of the compounds were not eligible for enrolment in the study. Patients with an open or incompletely healed wound at the affected site or those who had received any investigational medication in the previous three months or with continuing history of alcohol and/or drug abuse were also not included in the study.
Patients were not permitted to use any other systemic or topical treatment for acne vulgaris, peeling agents, abrasive cleansers, strong drying agents, astringents or irritant products (with aromatic and alcoholic agents) during the study period. Application of comedogenic cosmetics with a potential to exacerbate acne lesions was to be strictly avoided by the patients. Use of medications with neuromuscular blocking properties was also not permissible during the entire study period as clindamycin itself has neuromuscular blocking properties that may enhance the action of other neuromuscular blocking agents.
Study Procedures
Patients suffering from acne vulgaris of the face were evaluated as per the inclusion and exclusion criteria and underwent a thorough general physical and systemic examination to assess eligibility for participation. Eligible patients were randomized as per a centralized computer generated schedule to receive treatment with the test drug i.e. clindamycin nano emulsion gel formulation (Zyclin® Nanogel™; Cadila Healthcare Ltd., India) or the active control i.e. a marketed conventional clindamycin gel formulation (Clindac-A® Gel, Galderma International, India). Patients were instructed to apply a thin film of the study medication twice daily, with the fingertips, avoiding the eyes and lips, ensuring that the affected areas were clean and dry before application. The total duration of treatment was 12 weeks and the patients were followed-up on outpatient visits scheduled at weeks 4, 8 and 12 after the initiation of therapy.
The total number of lesions, including inflammatory lesions (papules, pustules, nodules and cysts) and non inflammatory lesions (open and closed comedones) were recorded to carry out efficacy assessments. Percentage reduction in the number of lesions as compared to the baseline was calculated. Acne severity grades/scores were assessed for acne severity as mentioned in [Table/Fig-1] [15,16]. The attainment of “clear™ or “almost clear™ grades of acne severity at the end of the treatment phase was defined as ‘treatment success’.
Acne severity grades [15,16]
| Grade | Score | Description |
|---|
| Clear | 0 | Normal-appearing, clear skin with no evidence of acne vulgaris |
| Almost clear | 1 | Rare non-inflammatory lesions present, with rare non-inflamed papules (papules must be resolving and may be hyperpigmented, although not pink-red) |
| Mild | 2 | Some non-inflammatory lesions are present, with few inflammatory lesions (papules/pustules only; no nodulocystic lesions) |
| Moderate | 3 | Non-inflammatory lesions predominate, with multiple inflammatory lesions evident: several to many comedones and papules/pustules, and there may or may not be one small nodulocystic lesion |
| Severe | 4 | Inflammatory lesions are more apparent, many comedones and papules/pustules, there may or may not be a few nodulocystic lesions |
| Very severe | 5 | Highly inflammatory lesions predominate, variable number of comedones, many papules/pustules and many nodulocystic lesions |
Primary efficacy variables were percentage reduction in the total number of lesions, inflammatory lesions, and non-inflammatory lesions at the end of therapy (i.e. week 12) and at each follow-up visit as compared to the baseline (i.e. week 0). Secondary efficacy variables were the treatment success rate and the degree of improvement in the acne severity grades at the end of therapy (i.e. week 12) as compared to the baseline (i.e. week 0).
The investigators documented adverse events on each of the scheduled visit, with date of onset, severity (mild, moderate or severe), its treatment, final outcome and duration of adverse event. The causality assessment of the study medication to the adverse event was evaluated as per the World Health Organization-Uppsala Monitoring Centre (WHO-UMC) criteria. The investigators’ rated the overall global assessment of tolerability at the end of the study on a four-point rating scale for each of the study medication.
Statistical Analysis
Efficacy and safety assessments were carried out in Intention-To-Treat (ITT) population comprising of the patients who received treatment with the study medication and attended at least one post-baseline assessment. The missing data values were completed by last observation carried forward (LOCF) procedure. Efficacy data of continuous variables are presented as mean, standard deviation (SD), & 95% confidence intervals (CI) and for ordinal/nominal variables as frequency (number) and percentage of patients along with 95% CIs. Student’s t-test, Chi-square test and Fischer’s Exact test were applied for statistical analysis by two-tailed assessments, according to the data characteristics. p-values <0.05 were considered as statistically significant.
Sample size was based on data (SD=37.8) from previous studies [16]. Allowing for a 20% drop out rate, it was estimated that a minimum of 100 patients in each group would be required to establish superiority of the nano-emulsion gel formulation as compared to the conventional formulation of clindamycin in terms of percentage reduction of inflammatory lesions at .05 significance level, with at least 80% power and a superiority margin of 5 %.
Results
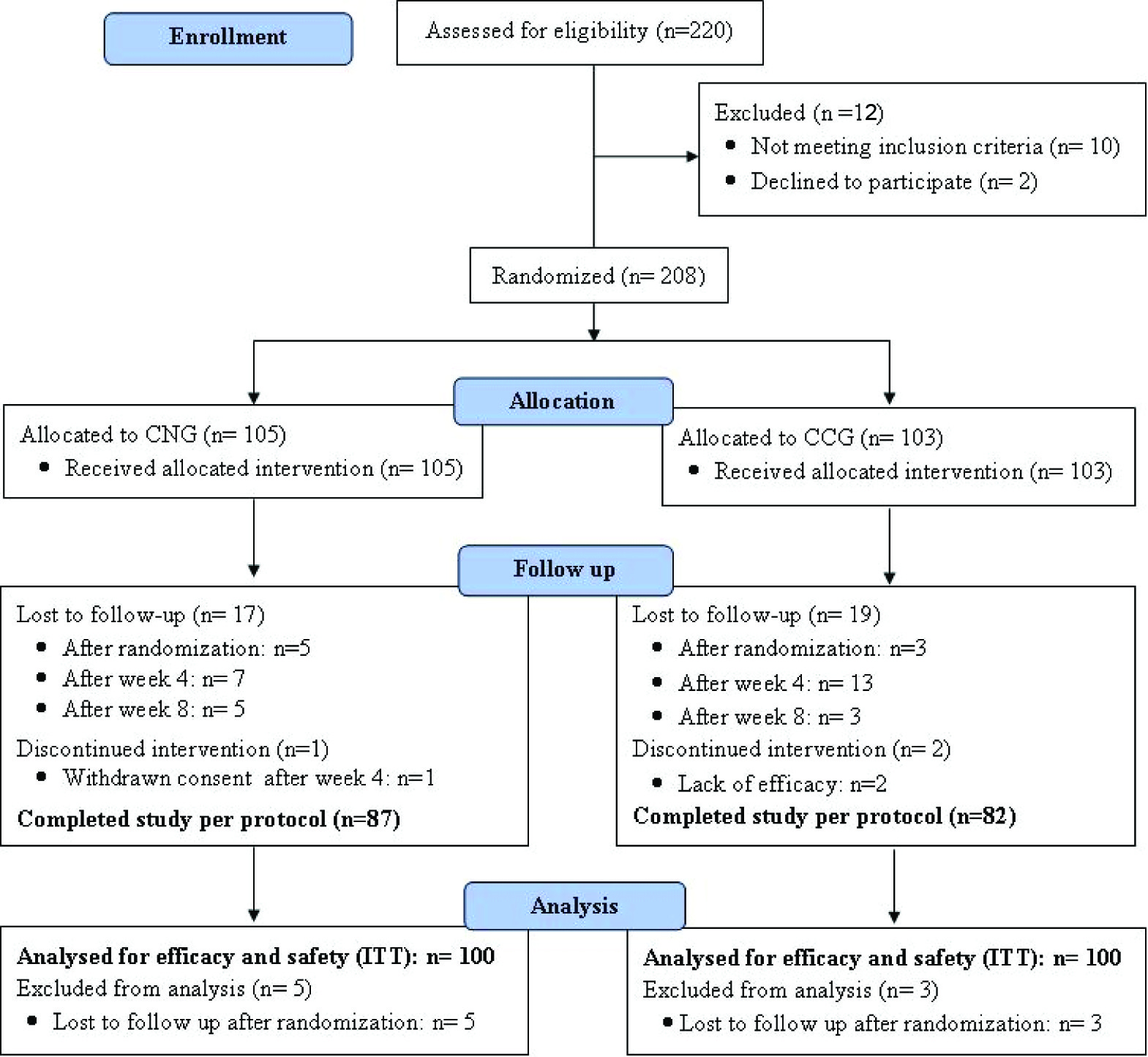
Two hundred and eight patients suffering from acne vulgaris of the face were enrolled in the study at seven different centres across the country. A total of 200 patients, 100 each in clindamycin nano-emulsion gel group (CNG group) and clindamycin conventional gel group (CCG group), completed at least one post-randomization visit and thus were included in the ITT analysis, while 169 patients completed the study as per protocol. The flow of the patients enrolled in the study is shown in [Table/Fig-2].
Flowchart of the patients enrolled in the study
Demographics and baseline characteristics
Both the treatment groups were comparable for the demographic characteristics. Proportions of male and female patients enrolled in each of the study groups were similar (p=0.479). The details of the demographic profile along with the baseline disease characteristics of the ITT population in each study group are shown in [Table/Fig-3]. Acne severity noted in patients in both the study groups was comparable as reflected by similar number of total, inflammatory, and non-inflammatory lesions as well as acne severity grades at the baseline.
Demographic and baseline disease characteristics of the patients [Mean ± SD/No.(%)]
| Characteristics | CNG group (n=100) | CCG group (n=100) | p-value |
|---|
| Age (year) | 21.9± 4.2 | 21.7 ± 3.9 | 0.74 |
| Sex | Females | 49 (49.0%) | 54 (54.0%) | 0.48 |
| Males | 51 (51.0%) | 46 (46.0%) |
| Height (cm) | 162.7 ± 7.9 | 161.4 ± 8.4 | 0.28 |
| Weight (kg) | 56.4 ± 8.6 | 57.4 ± 9.6 | 0.47 |
| Duration of illness (months) | 21.9 ± 23.7 | 20.0 ± 20.4 | 0.55 |
| Inflammatory Lesions | 11.5 ± 7.9 | 11.6 ± 7.9 | 0.97 |
| Non-Inflammatory Lesions | 16.6 ± 11.2 | 16.1 ± 10.3 | 0.75 |
| Total Lesions | 28.2 ± 15.0 | 27.7 ± 13.0 | 0.82 |
| Acne Severity Scores | 3.1 ± 0.7 | 3.0 ± 0.9 | 0.33 |
| Acne Severity | Grade 1 | 2 (2.0%) | 4 (4%) | 0.41 |
| Grade 2 | 8 (8.0%) | 25 (25%) |
| Grade 3 | 69 (69.0%) | 40 (40%) |
| Grade 4 | 18 (18%) | 28 (28%) |
| Grade 5 | 3 (3%) | 3 (3%) |
CNG = Clindamycin nano emulsion gel, CCG = Clindamycin conventional gel, SD = Standard Deviation
Efficacy assessments
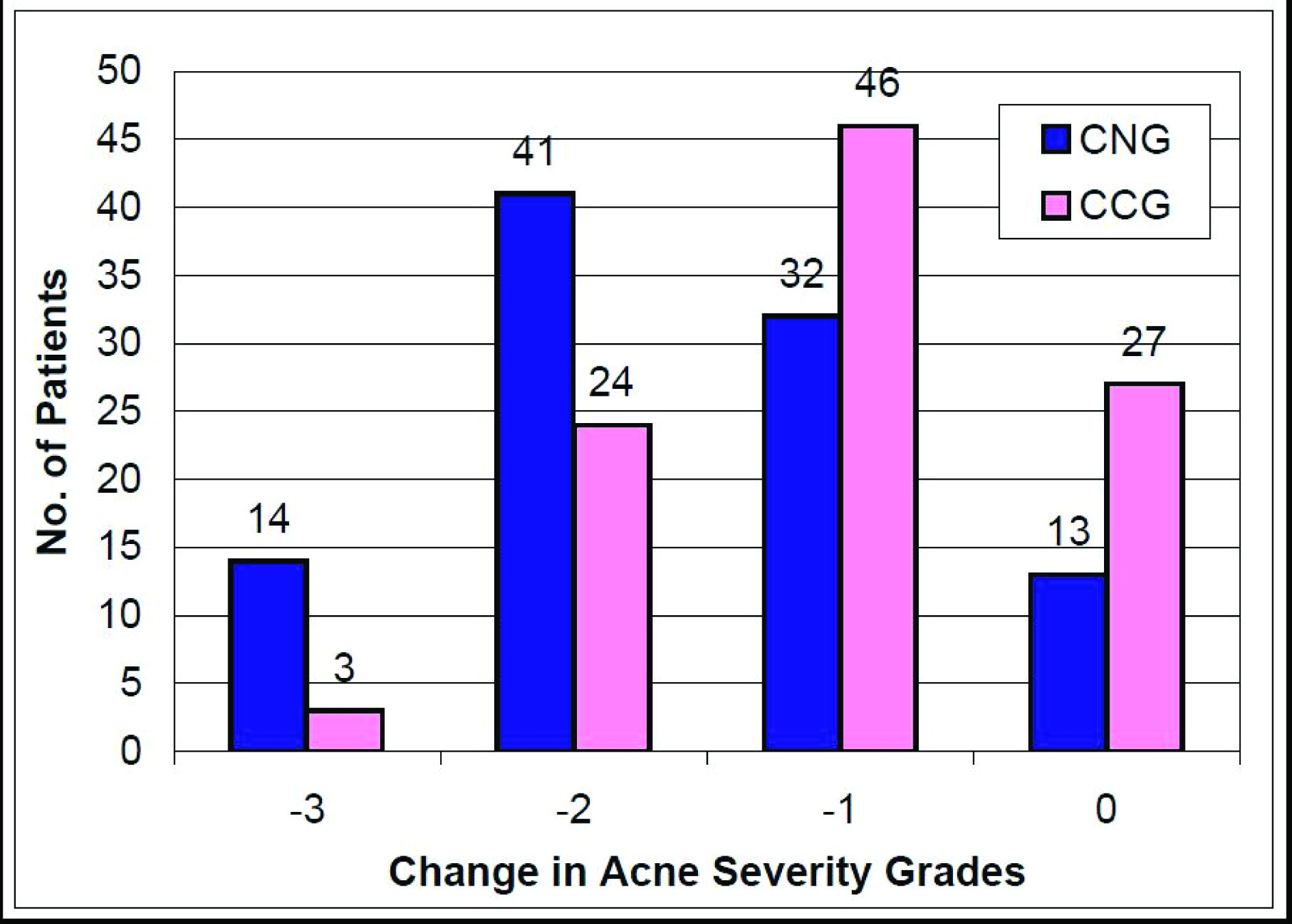
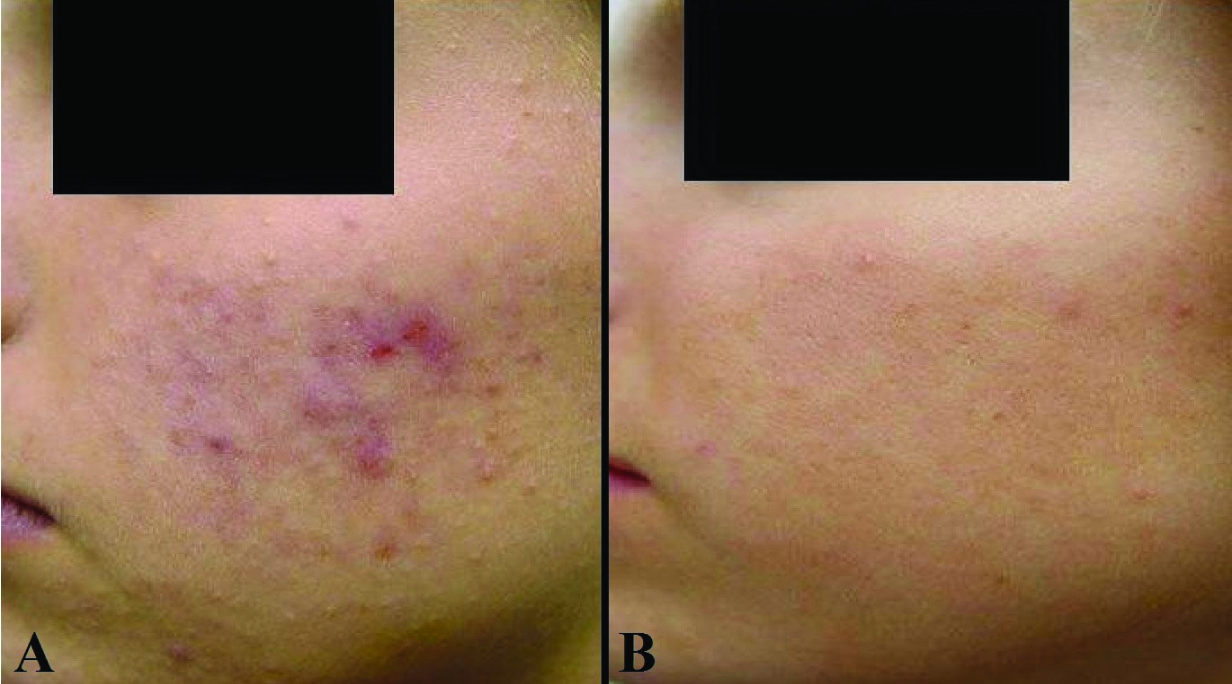
Acne lesions reduced after the initiation of therapy in all the enrolled patients in both the treatment groups during the course of the study. Significantly greater reductions in acne lesions started as early as four weeks after treatment and continued during all subsequent assessments till the end of the study at week 12 [Table/Fig-4,5]. Based on the change in the acne severity grades, 53% (43.2-62.8%) patients in CNG group achieved ‘treatment success’ as compared to 28.0% (19.8-36.2%) patients in CCG group (p<0.001). The reduction in the mean acne severity grades was significantly more (p<0.001) in CNG group (1.6 + 0.9) as compared to that in CCG group (1.0 + 0.8) at the end of the study. The change in the acne severity grades reported in each of the treatment groups at the end of the study (week 12) as compared to baseline is shown in [Table/Fig-6]. Fifty five percent (45.2-64.8%) patients had at least a two grade change (-3 or -2) in their acne severity at the end of treatment with CNG while 27% (18.9-35.1%) patients reported the same with CCG therapy (p<0.001). Further, one patient in the CNG group reported an increase in the severity of the acne lesions (completed the study); while two patients in the CCG group discontinued further participation in the study due to the lack of efficacy (one patient each after week 4 & week 8). The change in acne lesions as observed at baseline and at the end of treatment in a selected patient from the CNG group is shown in [Table/Fig-7].
The mean percentage reductions in total, inflammatory and non-inflammatory lesions during the course of the study as compared to the baseline CNG = Clindamycin nano emulsion gel, CCG = Clindamycin conventional gel, I-bars = SE (Standard Error of Mean), CNG group: n=100, CCG group: n=100, *p<0.05, $p<0.005, #p<0.001
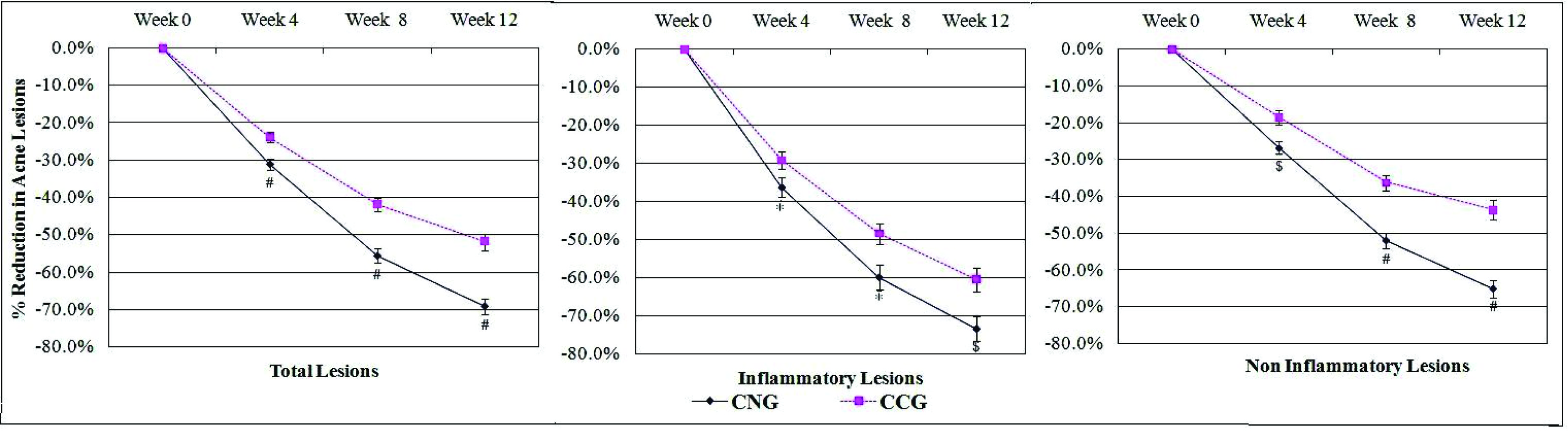
Mean percentage reductions in acne lesions in both the study groups at week 12 as compared to the baseline (Mean ± SD [95% CI])
| Characteristics | CNG group (n=100) | CCG group (n=100) | p-value |
|---|
| Inflammatory Lesions | 73.4 ± 31.7% [67.2-79.6%] | 60.6 ± 31.3% [54.3-66.9%] | <0.005 |
| Non-inflammatory Lesions | 65.1 ± 23.8% [60.4-69.8%] | 43.7 ± 26.5% [38.6-48.9%] | <0.001 |
| Total Lesions | 69.3 ± 21.5% [65.0-73.5%] | 51.9 ± 22.2% [47.6-56.2%] | <0.001 |
CNG = Clindamycin nano emulsion gel, CCG = Clindamycin conventional gel, SD = Standard Deviation, CI = Confidence Interval
The change in the acne severity grades at the end of the study (week 12) as compared to baseline in both the treatment groups CNG = Clindamycin nano emulsion gel, CCG = Clindamycin conventional gel, CNG group: n=100, CCG group: n=100
The effect of Clindamycin nano-emulsion gel on acne lesions after 12 weeks of treatment (A - baseline and B -12 weeks)
Tolerability assessments
Seven patients had 8 adverse events in the CNG group while 12 patients had 15 adverse events in the CCG group. Though not statistically significant (p=0.12), nano emulsion showed a trend towards better tolerability as compared to the conventional gel preparation. The list of adverse events reported in each of the treatment groups is given in [Table/Fig-8]. Further, all the adverse events reported in CNG group were of “mild™ intensity while; 5 (33.3% [9.5-57.2%]) of the 15 adverse events in CCG group were of “moderate™ intensity (p=0.122). All these adverse events in both the treatment groups had a “possible™ association with the respective study medication and resolved with/without symptomatic treatment during the course of the study.
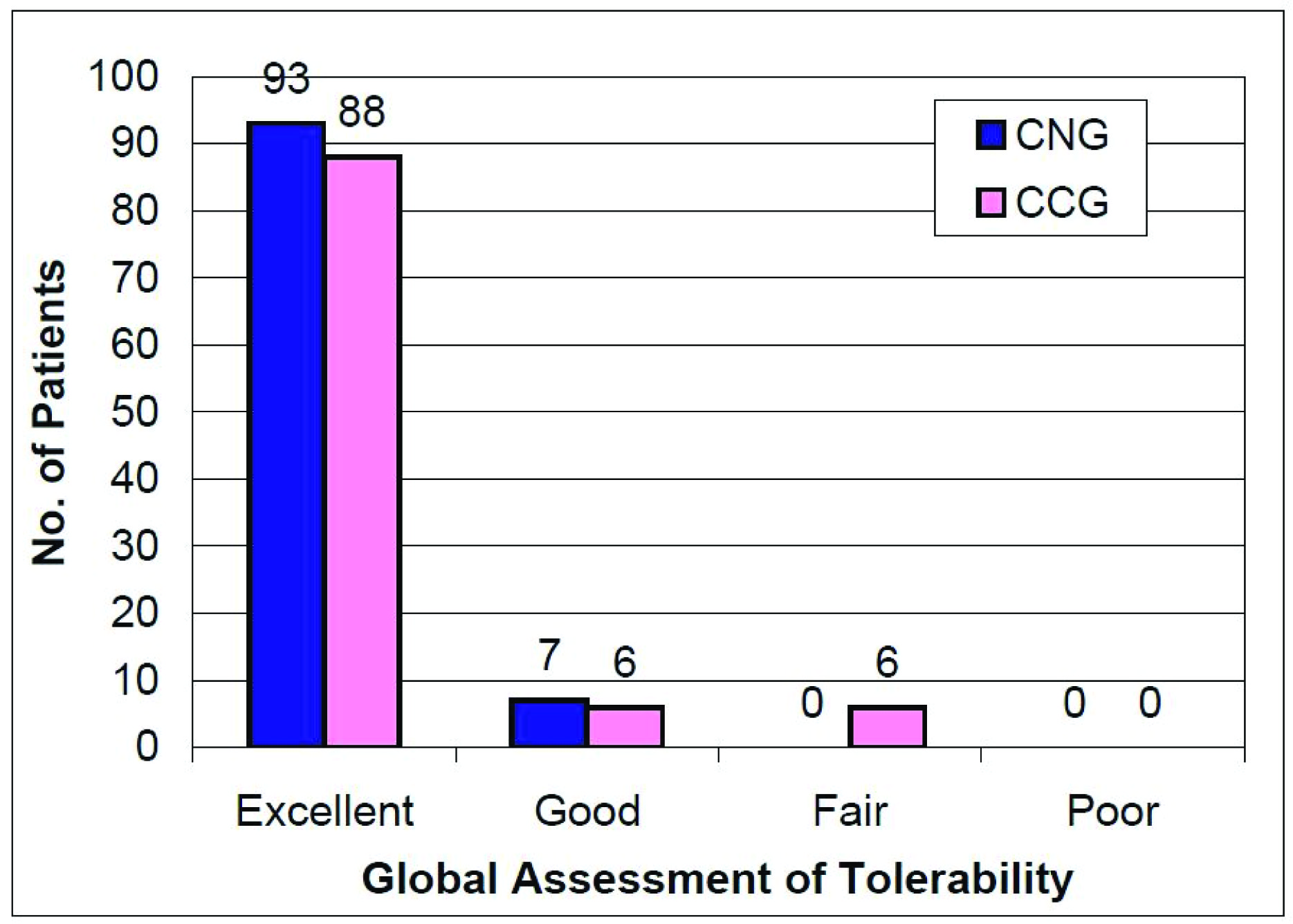
No “serious™ or “severe™ adverse event was reported during the entire course of the study in any of the two treatment groups. Further, none of the patients discontinued the study due to any adverse event in either of the study groups. The global assessment of tolerability as given to the study medication at the end of the study by the investigators is shown in [Table/Fig-9].
Adverse events reported in both the study groups [No.(%)]
| S. No | Nature | CNG group (n=100) | CCG group (n=100) | p-value |
|---|
| 1 | Local Irritation | 2 (2.0%) | 6 (6.0%) | 0.28 |
| 2 | Itching | 1 (1.0%) | 5 (5.0%) | 0.21 |
| 3 | Dryness of skin | 2 (2.0%) | 3 (3.0%) | 0.65 |
| 4 | Erythema | 3 (3.0%) | 1 (1.0%) | 0.62 |
| Total | 8 Adverse Events | 15 Adverse Events | 0.12 |
CNG = Clindamycin nano emulsion gel, CCG = Clindamycin conventional gel
The investigators’ overall assessment of tolerability at the end of the study CNG = Clindamycin nano emulsion gel, CCG = Clindamycin conventional gel, CNG group: n=100, CCG group: n=100
Discussion
The comparative evaluation of efficacy and safety of a novel nano-emulsion gel formulation of clindamycin with a similar conventional formulation was carried out in patients suffering from acne vulgaris of the face in this clinical study. The novel nano-emulsion gel formulation is reported to be more effective in reducing total acne lesions including both the inflammatory as well as the non-inflammatory lesions as compared to the conventional gel formulation. This nano-emulsion gel formulation also showed a trend towards better tolerability.
Nano-emulsion formulations are likely to have a better stability and longer shelf-life due to their stable thermodynamic properties [17].They increase the surface area of the drugs and thereby enhance their solubility as well as permeation through tissue barriers [18,19]. In fact, nano emulsions are shown to improve the active ingredients’ penetration into the epidermis, dermis and pilo-sebaceous units [20]. They also exert invitro and invivo direct bactericidal effects on several bacterial species including P. acnes [20,21]. Enhanced permeation into pilo sebaceous units through the hair follicles as well as by lateral diffusion to closed or infected comedones [21], and a synergistic action with topical antimicrobial agents is also reported [22]. Further, topical antimicrobials are generally associated with common mild local adverse events like erythema, scaling, dryness & burning sensation. Skin hydrating properties of the nano-emulsion formulations can improve the tolerability and acceptability of the topical preparation [23] and enhance treatment adherence resulting in better success rate of acne therapy.
The present study intended to assess the therapy with topical clindamycin for a period of 12 weeks during which optimum treatment response is generally obtained [24]. It is also in line with the current treatment recommendations which restrict the prolonged usage of the antibiotics in order to prevent the development of drug resistance [9]. Moreover, monotherapy with clindamycin was investigated to determine the comparative clinical effectiveness as well as safety of the nano-emulsion gel formulation in the absence of the other confounding treatment variables. Although open label design of the study can be affected by the investigator bias, which is inherent to all open label studies, active controlled studies are difficult to blind due to the obvious differences in the topical medications. Though, it can be achieved with complex study designs like double dummy technique, requirement of additional topical applications which can alter the treatment response and exert adverse influence on the patient compliance are the obvious disadvantages [25]. Due to the above intricacies, our active controlled study incorporated an open label design. A sample size of 208 patients was studied in this clinical trial but retrospective analysis showed that the primary efficacy variable achieved a power of more than 95% at the end of the study.
Significantly higher improvements in the mean percentages of inflammatory as well as total acne lesions were noticeable after CNG treatment as compared to CCG treatment, which were apparent as early as four weeks after treatment and persisted thereafter. Improvements in non-inflammatory lesions in CNG group were also significantly better at four weeks and thereafter. Treatment success rate at the end of study was almost twice with CNG treatment than with CCG. Similarly, the change in acne the severity of at least two grades was reported in double the number of patients receiving CNG than CCG. It was also noticeable that an incremental treatment response was observed throughout the course of the study and a “plateau™ effect was not noticeable till the end of 12 weeks of therapy. However, as per the current treatment recommendations, monotherapy with topical antibiotic preparations is not advisable for a treatment duration longer than 12 weeks [9].
Treatment response reported in the form of percentage reductions in acne lesions by several studies assessing clindamycin monotherapy has been variable. A recently published meta-analysis of the therapeutic efficacy of topical antimicrobial therapies has shown that monotherapy with clindamycin 1-1.2% formulations lead to ~45% reduction in inflammatory acne lesions, after 10-12 weeks of therapy [26]. The treatment response observed for inflammatory lesions after 12 weeks in our study in both the study groups has been reportedly larger (CNG: 73.4%, CCG: 60.6%) than that reported from this meta-analysis incorporating 14 clinical studies. On the other hand, Alirezai M et al., [27] and Zouboulis CC et al., [28] have reported the mean percentage reductions in inflammatory lesions (58-62%) similar to that reported in our study with CCG; while CNG has shown even better therapeutic response (73.4%). Thus, a significant increase in the efficacy in terms of resolution of inflammatory acne lesions is noteworthy with this novel nano emulsion gel formulation.
Further, the effect of topical clindamycin monotherapy in the reduction of non-inflammatory lesions of varying magnitude is consistently observed which ranges from 30-60% [26,28]. This response is incompletely understood and may involve the pathogenic role of P. acnes in comedone formation, [8,24] which is favourably altered by topical clindamycin therapy. CNG formulation has also shown better response with 65.1% reduction of these lesions as well, compared to 43.7% reduction with CCG.
Clindamycin nano emulsion gel formulation was well tolerated by the patients. The incidence and severity of adverse events were less as compared to the conventional formulation, which however did not reach any statistical significance. The investigators’ overall assessment of tolerability at the end of the study showed comparable safety of both the formulations. The commonly reported adverse events with topical clindamycin include erythema, dryness, burning/irritation and desquamation [29].
Thus, the results of the present study suggest that clindamycin nano emulsion gel formulation leads to a significantly better as well as faster response on the inflammatory acne lesions. Improved penetration of clindamycin into the infected pilo-sebaceous units with the synergistic bactericidal effects of the nano-emulsion itself could be responsible for these observations. Moreover, enhanced inhibitory effect of clindamycin on P. acnes along with its anti-inflammatory effects altering the incompletely understood natural history of comedone formation is a possible explanation for the improvement in non-inflammatory lesions. Better moisturizing properties of the nano-emulsion gel and optimized anti-inflammatory properties of clindamycin in the pilo-sebaceous glands could have lead to the favourable local tolerance observed. Thus, with better effectiveness and good tolerability, clindamycin nano-emulsion gel formulation holds a lot of promise for the management of acne vulgaris. It can aid in the prevention of bacterial resistance development by virtue of enhanced efficacy due to attainment of higher local concentration of the drug as well as the synergistic effects of the nano-emulsion vehicle.
Studies in various severity and morphological categories of acne with more robust study designs such as double blind, double dummy technique or split face comparisons are required for detailed elaboration of the comparative benefits of this novel nano-emulsion gel formulation. Clinical trials assessing combination therapy with other agents like retinoids or benzoyl peroxide for extended study durations of longer than 12 weeks can further envisage the therapeutic role of this novel formulation.
Conclusion
The results of this clinical trial for the treatment of acne vulgaris of the face suggest that clindamycin 1% nano-emulsion gel formulation is more efficacious than its conventional formulation and showed a trend towards better tolerability profile. Future studies can characterize and elaborate the therapeutic role of this nano-emulsion formulation in the management of acne vulgaris.