Introduction
Growth reflects a complex interplay of hormones as well as extrinsic influences and genetic factors. The pulsatality of growth hormone (GH) secretion under physiological conditions is controlled by a complex regulatory system primarily exerted by three hypothalamic neuroendocrine hormones acting as coupled biological oscillators, GH-releasing hormone (GHRH), somatostatin (GHIH) and ghrelin [1]. GHRH, is the principal stimulator of GH synthesis and secretion while somatostatin is a potent noncompetitive inhibitor of the release of GH and modulates the pituitary GH response to GHRH [1] . A third regulator of growth hormone secretion is ghrelin which has a marked growth hormone-stimulating activity.
Ghrelin; a type of Growth Hormone Secretagogue (GHS) was first isolated from the rat stomach in 1999 by Kojima and colleagues [2]. The name “ghrelin” is derived from “ghre” which means grow and “relin” which means release [3]. GHSs act through the GHS-receptor (GHS-R) which is a G protein-coupled receptor (GPCR) and stimulates the release of GH [2]. The isolation of ghrelin from stomach as an endogenous ligand of the growth hormone secretagogue receptor (GHSR) led to a reassessment of hormonal regulation of GH secretion.
Ghrelin cells in the oxyntic mucosa are closed types of cells with no continuity with the lumen of the gastrointestinal tract but lies near the capillary network in the lamina propria. Hence, they can respond to physical stimuli from the lumen, chemical stimuli from the basolateral site, or both and can thus function as endocrine cells delivering ghrelin to peripheral tissues that express GHS-R. Human ghrelin crosses the blood-brain barrier as an intact molecule [3,4]. The ghrelin receptor (GHS-R1a) is conserved across different vertebrate species of mammals, birds and fishes [5-7]. Transcripts for GHSR 1A are expressed at low levels in many tissues, but mostly in the arcuate and ventromedial nuclei of the hypothalamus, pituitary hippocampus and gastrointestinal tract [8,9].
This review highlights the interaction of ghrelin with the hypothalamic hormones; GHRH and somatostatin to regulate the secretion of SectionGH and intends to explore the interesting aspect of the possible physiological role of the ghrelin-pituitary-GH axis linkage system.
Methodology
The search was performed in electronic databases (MEDLINE, EMBASE, Cochrane, Google scholar). The electronic database search was conducted using the following MeSH terms and keyword combinations: (“ghrelin”[MeSH Terms] OR “ghrelin”[All Fields]) AND (“growth “[Subheading] OR (“growth”[All Fields] AND “development”[All Fields]) OR “growth”[All Fields] OR “growth and development”[MeSH Terms]). Hand searching was also carried out. Articles were selected on the basis of the abstracts, before examining the full text. The reviewers were not blinded to the journal, authors, or institution of the publications. All applicable references were examined and scrutinized by 2 reviewers.
Data extraction: Data extraction was performed by two reviewers on the full texts using a predefined extraction sheet available from the authors. These data included ghrelin/history, ghrelin/physiology, ghrelin/chemistry, ghrelin/ biosynthesis, ghrelin/secretion, ghrelin/pharmacokinetics, ghrelin/blood, ghrelin/analogs and derivatives, ghrelin/administration and dosage, ghrelin/agonists, ghrelin/antagonists and inhibitors, ghrelin/diagnostic use, ghrelin/deficiency ghrelin/therapeutic use, ghrelin/drug effects.
Inclusion criteria: Clinical trials (Randomised and non-randomised trials), review articles, systematic reviews, conference proceedings were included in the study. Animal studies were also included.
Exclusion criteria: Studies published in non-indexed journals and in any language other than English were excluded from the study.
Ghrelin gene and synthesis of ghrelin
In humans, the ghrelin gene is located on chromosome 3, at locus 3p25 –2 and is made up of five exons and 3 introns [10]. The first exon is very short (containing only 20 bp) and codes the 5′ region [1,11,12]. Ghrelin gene exhibits polymorphism and is not associated with any changes in circulating ghrelin concentration [13]. mRNA from the ghrl gene codes for a 117 amino acid peptide called preproghrelin which is then cleaved to produce proghrelin. Finally, a 28-amino acid acylated ghrelin peptide is produced by protease cleavage and acyl-modification at Ser 3 with an n-octanoyl [11] . Post-transductional mechanisms produce different isoforms of ghrelin, either acylated at Ser with a fatty acid, or have a deletion of the C-terminal Arginine at position 28 [13] .
Ghrelin cells: Ghrelin originates in a distinct round to ovoid endocrine cells called “grl cells” [14]which are referred as P/D1 cell in humans and X/A-like cell in rats due to their similarity with the A-cell of the rat pancreas. Ghrelin cells accounts for 23% of chromogranin A-immunoreactive endocrine cells. However, the morphological characteristics and thorough distribution of ghrelin cells in the gastrointestinal tract has not been defined. Ghrelin is also produced in small amounts by other organs like heart, lung, lymphatic tissue, kidney, adrenal glands, thyroid gland, pancreas, gonads, some neoplastic tissues and cancer-cell lines [15-17] . Ghrelin- immunoreactive neurons are present in few regions of the brain like the arcuate and the ventromedial nucleus of the hypothalamus, cerebellum and brainstem [12,18-20] . Ghrelin is also present in placenta along GH, GHRH, somatostatin and insulin-like growth factor 1 (IGF1) which shows that it is also involved in fetal growth [21] .
Structure of Ghrelin: A total molecular mass of human ghrelin is 3.370 Kilodalton and the molecular formula is C 149 H 249 N 47 O 42. It is the first and only known peptide hormone in which the hydroxyl group of one of its serine residues is acylated by a fatty acid n-octanoic acid [11,22] . Ghrelin has a hydrophobic moiety: an octanoylated linear chain and the ester bond linking alkyl chain to the serine side chain. The synthetic peptide shows same biological activities as the isolated purified material. Structurally and functionally, ghrelin resembles gut hormone motilin and may have evolved from common ancestral gene [11] . They are therefore now considered to be members of the new motilin-ghrelin peptide superfamily [11] . The human ghrelin and human motilin shows 36% identity.
Ghrelin in different species: Ghrelin are highly conserved among species. The amino acid sequence at positions 11 and 12 is different in rat and human ghrelin and they are equally potent [2,23] n-octanoylated ghrelin and the shorter form human Ghrelin (hGhr18) is necessary for the GH-releasing effect of ghrelin and the longer forms of Ghrelin are active in vivo [24].
Forms and derivatives of Ghrelin: According to the different types of acylation at Ser3, these peptides could be classified into four groups namely octanoylated (C8:0), decanoylated (C10:0), decenoylated (C10:1) and nonacylated [25]. All peptides are obtained from the same precursor of ghrelin by two alternative pathways and are either 27 or 28 amino acids in length. As in rats, a 28-amino acid peptide octanoylated at Ser3 is the major active form of human Ghrelin. However, the ratio of circulating desacyl ghrelin to acyl ghrelin is 9: 1 while in the human stomach, the ratio is around 1:3 [26,27].
The characteristic feature of AG is the presence of n-octanoyl ester at serine-3 position. The acylation of ghrelin promoted by Ghrelin O-acyltransferase (GOAT) [27] is essential not only for binding with GHS-R1a but also for stimulation of GH secretion [28,29]. Unacylated ghrelin cannot bind and activate the GHS-R 1a and hence is devoid of any effects on endocrine axes. However, it shares other functions of AG through interaction with an unidentified GHS receptor that is different from the classical GHS1a receptor [30,31]. At first UAG was considered an inactive form of ghrelin but now studies have demonstrated that UAG plays a beneficial role in improvement of pancreatic β-cell function and survival [32], and in cardiovascular function [33].
Des-Gln14-ghrelin is an endogenous ligand for the GHSR isolated from the mucosa of the stomach and shares similar hormonal functions as ghrelin [2].
Ghrelin O-acyltransferase (GO AT): GOAT has been regarded as an orphan member of a family of membrane-bound O- acyltransferase enzymes (MBOATs) and is the only known enzyme that has a capacity to acetylate ghrelin. This membrane-bound enzyme can transfer octanoate to serine-3 of ghrelin from octanoyl CoA [34,35]. Since the acyl modification is important for majority of actions of ghrelin, GOAT has emerged as a vital enzyme of interest for research. GOAT expression is tissue specific.
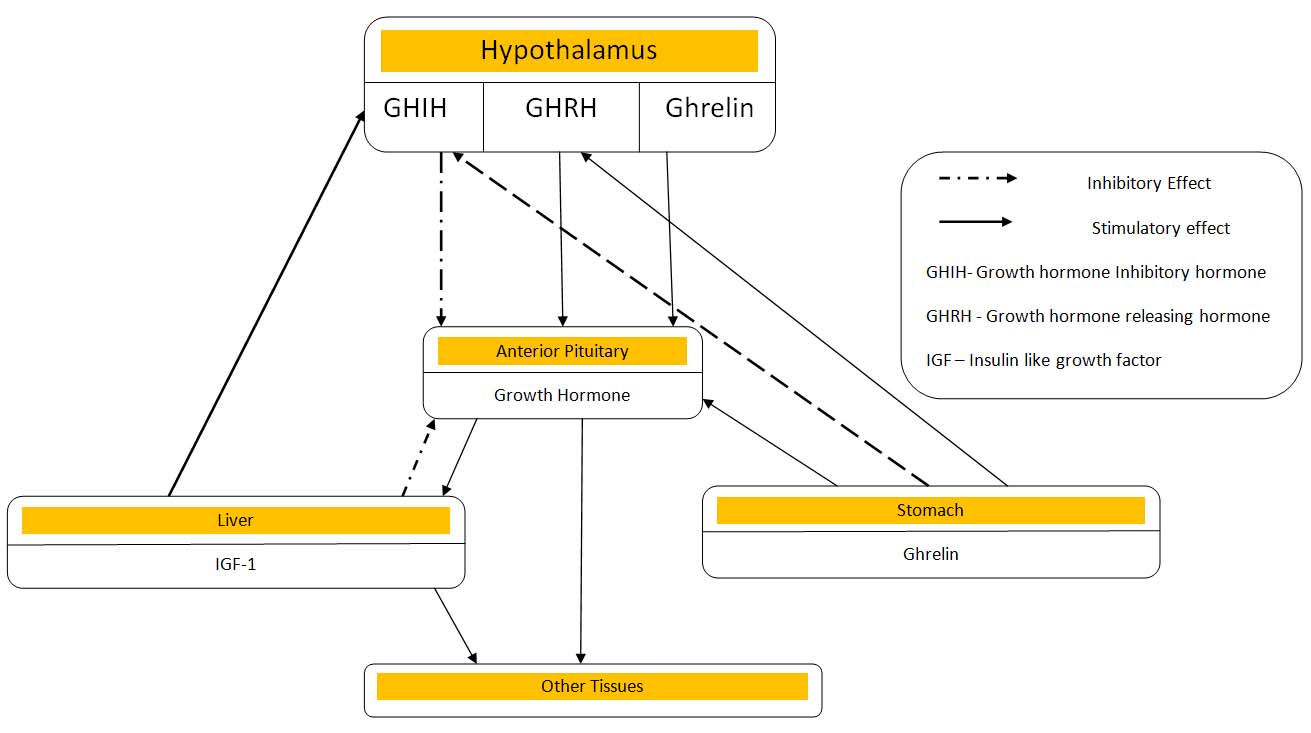
Mechanism of action of ghrelin: Most of the regulation of secretion of growth hormone is mediated through GHRH rather than through GHIH i.e. somatostatin. In the somatotroph cells of the anterior pituitary gland, GHRH stimulates the release of GH by binding to the GHRH receptor to increase the cAMP levels. On the other hand, GHSs stimulates the release of GH binding through the GHSR-1a or ghrelin receptor to increase intracellular calcium ion levels in the cell [Table/Fig-1] [11]. Receptor–ligand interaction is just the beginning of the cell response. This event is transduced into secondary responses within the cell that can be divided into four broad categories: (1) ion channel activation, (2) G-protein activation, (3) activation of enzyme activity within the cell, or (4) direct activation of transcription/ initiate changes in cell function. The extracellular ligands are called “first messengers” and the intracellular mediators are called “second messengers.” Second messengers bring about many short-term changes in cell function by triggering exocytosis, but they also can lead to long term changes like alteration of transcription of genes to stimulate the synthesis of new growth hormone.
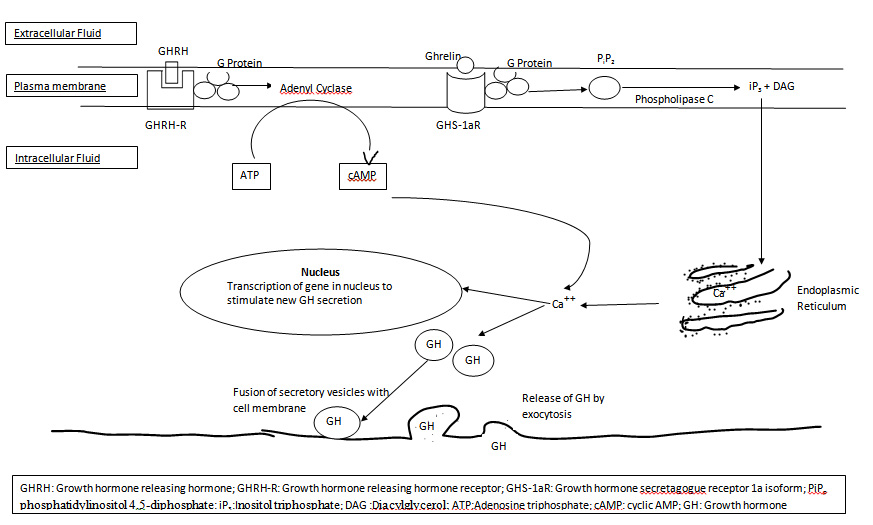
Ghrelin produced peripherally acts on the pituitary and the hypothalamus via the modulation of the vagus nerve. The studies have proposed that ghrelin acts on both the somatotrophs of the anterior pituitary gland and GHRH-secreting neurons and on GHIH-secreting neurons (opposes the action of somatostatin) in the hypothalamus via GHSR 1a, a G protein-coupled receptor [36,37]. Once ghrelin binds with GHSR-1a, it gets activated which further activates phospholipase C (PLC) attached to the inner portions of the receptors . PLC has at least eight isoforms. PLC isoforms catalyses the hydrolysis of some cell membrane phos- pholipids especially phosphatidylinositol 4,5-diphosphate (PIP2) into inositol triphosphate (IP3) and diacylglycerol (DAG) which act as two different second messengers. The IP3 binds the IP3 receptor which is a ligand-gated Ca2+ channel of the endoplasmic reticulum and triggers the release of Ca2+ into the cytoplasm. Additional calcium also enters from the extracellular medium via voltage-operated L-type channels. The calcium ions then act as second messengers and causes contraction of smooth muscles in the cell ghrelinand causes secretory changes in the cell. Calcium ions interact with the vesicular membrane and causes fusion of the growth hormone secretory vesicles with the cell membrane, followed by exocytosis i.e. extrusion of the GH outside the cell [11] . It also increases transcription of genes in the nucleus to stimulate the synthesis of new growth hormone [Table/Fig-2].
Growth hormone, thus secreted promotes synthesis of new proteins while at the same time conserves the proteins already present in the cells. However; like the secretion of other anterior pituitary hormones, growth hormone secretion is under feedback control. It acts on the hypothalamus to antagonize GHRH release. Growth hormone also increases circulating IGF-I, and IGF-I in turn exerts a direct inhibitory action on growth hormone secretion from the pituitary. It also stimulates somatostatin secretion [Table/Fig-1].
Action of ghrelin on pituitary and hypothalamus: In humans, GHS-induced GH release is dependent on a functional endogenous GHRH system [38]. As revealed by quantitative analysis of double-labelled cells, the GHS-R are expressed in about one fourth of GHRH mRNA containing neurons in arcuate (ARC) and ventromedial nucleus (VMN) of the hypothalamus thereby suggesting that ghrelin may influence GH secretion through interaction with the GHS-R and that the release of GHRH into hypophyseal portal blood may also be influenced by ghrelin. There is a dual action of GHS and ghrelin on the hypothalamus [9] . They act by increasing the electrical activity and c-fos expression in a subpopulation of some of the GHRH-producing neurons cells in the arcuate nucleus [39].
Stomach-ghrelin-pituitary-GH axis links food intake to regulation of secretion of GH. Exogeneously administered GHS predominantly antagonizes somatostatin and stimulates the release of GHRH thereby increases GH release from somatotrophs of anterior pituitary. This elevated GH provides a negative feedback to the hypothalamus to start a fresh cycle by enhancing the somatostatin tone on GHRH-containing neurons. This leads to the inhibition of GHRH and GH release and resets the oscillators.
Amount of GH released after administration of ghrelin is much greater than that induced by maximal dosages of GHRH thereby indicating that ghrelin is more potent in releasing GH than the unnatural GHRP/GHSs. Ghrelin and GHRH when administered simultaneously has a synergistic effect on the secretion of GH. Synergistic effect on GH release is also observed by co-administration of GHSs and synthetic ghrelin agonists. This indicates that GHRH is essential for the release of GH. Since GH secretion is maintained in the presence of GHRH and GH-releasing activity of ghrelin in vivo is dependent on endogenous GHRH; the primary feed-forward regulator of GH secretion is possibly GHRH. Ghrelin can thus be best regarded as a helper and/or modulator of GHRH in the regulation of secretion of GH [40].
Effect of in-vivo and in-vitro administration of ghrelin on GH secretion: Ghrelin stimulates strong increase in circulating GH levels both invitro and invivo in a dose-dependent manner [41,42]. Intravenous administration of ghrelin stimulates GH release [43] which peaks at 5–15 min and returns to basal levels after 1h. Ghrelin administered intravenously is more potent than GH-releasing hormone (GHRH) in stimulating the release of GH in anaesthetised or freely moving rats, and in humans [44,45]. Intracerebroventricular injection appears to be a more potent route of delivery than intravenous administration. Together, these invivo assays confirms that ghrelin is a potent GH-releasing peptide.
Ghrelin stimulates GH secretion invivo as well as from both pituitary cells in culture [42]. In contrast to the in-vivo studies, in vitro studies using primary pituitary cells shows that ghrelin stimulates the release of GH, but does not alter the release of other four pituitary hormones [46], thereby suggesting that ghrelin acts directly on the somatotrophs of the pituitary. Also, in contrast to the invivo studies, invitro studies shows that GH-releasing activity of ghrelin is similar with or less than that induced by GHRH [47].
Observations of these invivo and invitro studies suggests that ghrelin is a specific endogenous ligand for the GHS receptor and provides a proof of the occurance of a GHS–GHS receptor signalling system in the regulation of secretion of GH [48].
Therapeutic efficacy of ghrelin: Though studies have suggested that ghrelin is a powerful pharmacological agent for stimulating the release of GH due to their pharmacokinetic properties and high bioavailability following oral or parenteral administration, the physiological importance of the ghrelin in regulating GH still remains unclear. Several ghrelin analogs have been shown to be useful both as a treatment modality for GH deficiency and as an endocrine diagnostic tool. Co-administration of ghrelin with GHRH and other drugs is currently being investigated to develop novel diagnostic and therapeutic tools [49].
Regulation of growth hormone secretion
Mechanism of secretion of growth hormone by ghrelin
Conclusion
Ghrelin, a bioactive peptide is a natural endogenous ligand of the growth hormone secretagogue receptor 1a (GHSR1a). Under physiological conditions, ghrelin administered either centrally or peripherally, exerts a potent, time-dependent stimulation of pulsatile secretion of GH. An intact endogenous GHRH system is required for the GH response to ghrelin.
[1]. LV Muller EE, D Cocchi, Neuroendocrine control of growth hormone secretion.Physiol Rev. 1999 79:511-607. [Google Scholar]
[2]. HH Kojima M, Y Date, M Nakazato, H Matsuo, K Kangawa, Ghrelin is a growth-hormone-releasing acylated peptide from stomach.Nature. 1999 402(6762):656-60. [Google Scholar]
[3]. GY Alker AK, WM Park, JM Zigman, I Sakata, Expression of Serum Retinol Binding Protein and Transthyretin within Mouse Gastric Ghrelin Cells.PLoS ONE. 2013 8(6):e64882 [Google Scholar]
[4]. HT Stengel A, M Goebel-Stengel, V Lembke, A Ahnis, U Elbelt, Ghrelin and NUCB2/nesfatin-1 are expressed in the same gastric cell and differentially correlated with body mass index in obese subjects.Histochemistry and cell biology. 2013 139(6):909-18. [Google Scholar]
[5]. WJ Cheung CK, Role of Ghrelin in the Pathophysiology of Gastrointestinal Disease.Gut and liver. 2013 7(5):505-12. [Google Scholar]
[6]. HH Kaiya H, K Kangawa, M Miyazato, Determination of nonmammalian ghrelin.Methods in enzymology. 2012 514:75-87. [Google Scholar]
[7]. KK Kaiya H, M Miyazato, Ghrelin receptors in non-Mammalian vertebrates.Frontiers in Endocrinology. 2013 :4-81. [Google Scholar]
[8]. GJ Dockray, HS Sharma, Enteroendocrine cell signalling via the vagus nerve.Curr Opin Pharmacol. 2013 13(6):954-58. [Google Scholar]
[9]. L Xu, Y Gong, H Wang, X Sun, F Guo, S Gao, The stimulating effect of ghrelin on gastric motility and firing activity of gastric-distension-sensitive hippocampal neurons and its underlying regulation by the hypothalamus. Exp Physiol. 2014 99(1):123-35. [Google Scholar]
[10]. CC Seim I, C Adrian, Herington Chopin Lisa K, Revised genomic structure of the human ghrelin gene and identification of novel exons, alternative splice variants and natural antisense transcripts.BMC Genomics. 2007 8:298 [Google Scholar]
[11]. KK Masayasu Kojima, Ghrelin: Structure and Function.Physiol Rev. 2005 85(2):495-522. [Google Scholar]
[12]. CJ Bowers C, M Momany, K Folkers, Effect of the enkephalins and enkephalin analogs on release of pituitary hormones, in vitro. In: Molecular Endocrinology, edited by G.MacIntyre and H. Szelke. Amsterdam: Elsevier/North-Holland.:287-292. [Google Scholar]
[13]. AR Vivenza D, Castellino C, Bellone S, Petri A, Vacca G, Aimaretti G, Ghrelin gene polymorphisms and ghrelin, insulin, IGF-I, leptin and anthropometric data in children and adolescents.European Journal of Endocrinology. 2004 151:127-33. [Google Scholar]
[14]. J H. Body Composition in Early Growth: Lessons from Domestic Animals.European Journal of Pediatrics. 2006 165:1-389. [Google Scholar]
[15]. TJ Cao Y, T Yang, H Ma, D Yi, C Gu, Cardioprotective Effect of Ghrelin in Cardiopulmonary Bypass Involves a Reduction in Inflammatory Response.PLoS ONE. 2013 8(1):505-12. [Google Scholar]
[16]. LJ Kim YS, TH Lee, JY Cho, JO Kim, WJ Kim, Plasma levels of acylated ghrelin in patients with functional dyspepsia.World Journal of Gastroenterology : WJG. 2012 18(18):2231-37. [Google Scholar]
[17]. KK Miki K, N Nagaya, M Nakazato, H Kimura, S Murakami, Ghrelin Treatment of Cachectic patients with Chronic Obstructive Pulmonary Disease: A Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial.PLoS ONE. 2012 7(5):e35708 [Google Scholar]
[18]. SO Cabral A, JM Zigman, M Perello, Ghrelin Indirectly Activates Hypophysiotropic CRF Neurons in Rodents.PLoS ONE. 2012 7(2):e31462 [Google Scholar]
[19]. LX Cui RJ, SM Appleyard, Ghrelin Inhibits Visceral Afferent Activation of Catecholamine Neurons in the Solitary Tract Nucleus. The Journal of neuroscience : the official. Journal of the Society for Neuroscience. 2011 31(9):3484-92. [Google Scholar]
[20]. RS Emanuel AJ, Hindbrain Catecholamine Neurons Modulate the Growth Hormone But Not the Feeding Response to Ghrelin.Endocrinology. 2010 151(7):3237-46. [Google Scholar]
[21]. LS Martin JR, J McGrath, M Shanabrough, TL Horvath, HS Taylor, Maternal Ghrelin Deficiency Compromises Reproduction in Female Progeny through Altered Uterine Developmental Programming.Endocrinology. 2011 152(5):2060-66. [Google Scholar]
[22]. AT Kaiya H, T Ichikawa, N Amiya, K Matsuda, K Kangawa, Determination of Ghrelin Structure in the Barfin Flounder (Verasper moseri) and Involvement of Ingested Fatty Acids in Ghrelin Acylation.Frontiers in Endocrinology. 2013 4:117-60. [Google Scholar]
[23]. T I. Variety of acyl modifications in mammalian ghrelins.Methods enzymology. 2012 514:63-73. [Google Scholar]
[24]. ZP Tolle V, C Tomasetto, MC Rio, J Epelbaum, MT Bluet-Pajot, In vivo and in vitro effects of ghrelin/motilin-related peptide on growth hormone secretion in the rat.Neuroendocrinology. 2001 73(1):54-61. [Google Scholar]
[25]. H Hosoda, M Kojima, T Mizushima, S Shimizu, K Kangawa, Structural divergence of human ghrelin. Identification of multiple ghrelin-derived molecules produced by post-translational processing.J Biol Chem. 2003 278(1):64-70. [Google Scholar]
[26]. MK Patterson M, CW le Roux, CW Ghatei, SR Bloom, Characterization of ghrelin-like immunoreactivity in human plasma.Journal of Clinical Endocrinology and Metabolism. 2005 90(4):2205-11. [Google Scholar]
[27]. JP Seim I, L de Amorim, CM Walpole, J Fung, EJ Whiteside, Ghrelin O-acyltransferase (GOAT) is expressed in prostate cancer tissues and cell lines and expression is differentially regulated in vitro by ghrelin.Reproductive biology and endocrinology : RB & E. 2013 11:70Epub 2013/07/25 [Google Scholar]
[28]. CM Camina JP, D Micic, M Pombo, F Kelestimur, C Dieguez, Regulation of ghrelin secretion and action. Endocr. 2003 22(1):5-12.Epub 2003/11/12 [Google Scholar]
[29]. VS Colinet FG , B Charloteaux, A Eggen, N Gengler, B Renaville, Genomic location of the bovine growth hormone secretagogue receptor (GHSR) gene and investigation of genetic polymorphism.Animal biotechnology. 2009 20(1):28-33.Epub 2009/01/23 [Google Scholar]
[30]. FF Perdona E, A Buson, FM Sabbatini, C Corti, M Corsi, Pharmacological characterization of the ghrelin receptor antagonist, GSK1614343 in rat RC-4B/C cells natively expressing GHS type 1a receptors. European journal of pharmacology. 2011 650(1):178-83. [Google Scholar]
[31]. BA Muccioli G, R Granata, M Papotti, E Ghigo, Heterogeneity of ghrelin/growth hormone secretagogue receptors. Toward the understanding of the molecular identity of novel ghrelin/GHS receptors.Neuroendocrinology. 2007 86(3):147-64. [Google Scholar]
[32]. VM Granata R, F Settanni, C Gauna, C Ghe, M Annunziata, Unacylated ghrelin and obestatin increase islet cell mass and prevent diabetes in streptozotocin-treated newborn rats.Journal of molecular endocrinology. 2010 45(1):9-17. [Google Scholar]
[33]. TA Togliatto G, P Dentelli, A Baragli, A Rosso, R Granata, Unacylated ghrelin rescues endothelial progenitor cell function in individuals with type 2 diabetes.Diabetes. 2010 59(4):1016-25.Epub 2010/01/14 [Google Scholar]
[34]. HY Barnett BP, MS Taylor, H Kirchner, PT Pfluger, Glucose and weight control in mice with a designed ghrelin O-acyltransferase inhibitor.Science. 2010 330:16989-92. [Google Scholar]
[35]. FP Moussouttas M, M Rosenblatt, Pulmonary arterio- venous malformations: cerebral ischemia and neurologic manifes- tations.Neurology. 2000 55:959-64. [Google Scholar]
[36]. AD Gomes I, JH Wardman, A Gupta, K Gagnidze, RM Rodriguiz, editor. GPR171 is a hypothalamic G protein-coupled receptor for BigLEN, a neuropeptide involved in feeding.Proceedings of the National Academy of Sciences of the United States of America. 2013 [Google Scholar]
[37]. KK Mary S, M Damian, G Gaibelet, H Orcel, P Verdie, Heterodimerization with Its Splice Variant Blocks the Ghrelin Receptor 1a in a Non-signaling Conformation: A study with a purified heterodimer assembles into lipid discs. The Journal of biological chemistry. 2013 288(34):24656-65. [Google Scholar]
[38]. B Dominguez, T Avila, J Flores-Hernandez, G Lopez-Lopez, H Martinez-Rodriguez, R Felix, Up-regulation of high voltage-activated Ca(2+) channels in GC somatotropes after long-term exposure to ghrelin and growth hormone releasing peptide-6.Cellular and molecular neurobiology. 2008 28(6):819-31. [Google Scholar]
[39]. CH Sirinathsinghji DJ, R Hopkins, M Trumbauer, R Heavens, M Rigby, RG Smith, Induction of c-fos mRNA in the arcuate nucleus of normal and mutant growth hormone-deficient mice by a synthetic non-peptidyl growth hormone secretagogue.Neuroreport 1995 6(15):1989-92. [Google Scholar]
[40]. A Mano-Otagiri, T Nemoto, A Sekino, N Yamauchi, Y Shuto, H Sugihara, Growth hormone-releasing hormone (GHRH) neurons in the arcuate nucleus (Arc) of the hypothalamus are decreased in transgenic rats whose expression of ghrelin receptor is attenuated: Evidence that ghrelin receptor is involved in the up-regulation of GHRH expression in the arc. Endocrinology. 2006 147(9):4093-103. [Google Scholar]
[41]. BC Bitar K , D Coy, Effect of substance P, bombesin antagonists on the release of growth hormone by GHRP and GHRH. Biochem.Biophys. Res. Commun. 1991 180:156-61. [Google Scholar]
[42]. FR Sattler, Growth hormone in the aging male.Best practice and research Clinical endocrinology and metabolism.. 2013 27(4):541-55. [Google Scholar]
[43]. F Strasser, TA Lutz, MT Maeder, B Thuerlimann, D Bueche, M Tschop, Safety, tolerability and pharmacokinetics of intravenous ghrelin for cancer-related anorexia/cachexia: a randomised, placebo-controlled, double-blind, doublecrossover study.British journal of cancer. 2008 92(2):300-08. [Google Scholar]
[44]. LB de Sa, SO Nascif, P Molica, JG Vieira, U Dib, Effects of ghrelin, growth hormone-releasing peptide-6, and growth hormone-releasing hormone on growth hormone, adrenocorticotropic hormone, and cortisol release in type 1 diabetes mellitus.Metabolism: clinical and experimental. 2010 59(10):1536-42. [Google Scholar]
[45]. T Ida, Variety of acyl modifications in mammalian ghrelins.Methods in enzymology. 2012 514:63-73. [Google Scholar]
[46]. VJ Redman LM, J Rood, SR Smith, D Williamson, E Ravussin, The effect of caloric restriction interventions on growth hormone secretion in nonobese men and women.Aging cell. 2010 9(1):32-39. [Google Scholar]
[47]. S FR, M Miyazato, Growth hormone in the aging male.Best practice and research Clinical endocrinology and metabolism. 2013 27(4):541-55. [Google Scholar]
[48]. CA Carreira MC, S Andrade, MP Monteiro, FF Casanueva, Ghrelin as a GHreleasing factor.Endocrine development. 2013 25:49-58. [Google Scholar]
[49]. RG Raimondo S, S Geuna, D Pascal, S Reano, N Filigheddu, Ghrelin:A Novel Neuromuscular Recovery Promoting Factor? International review of neurobiology. 2013 108c:207-21. [Google Scholar]