Case Report
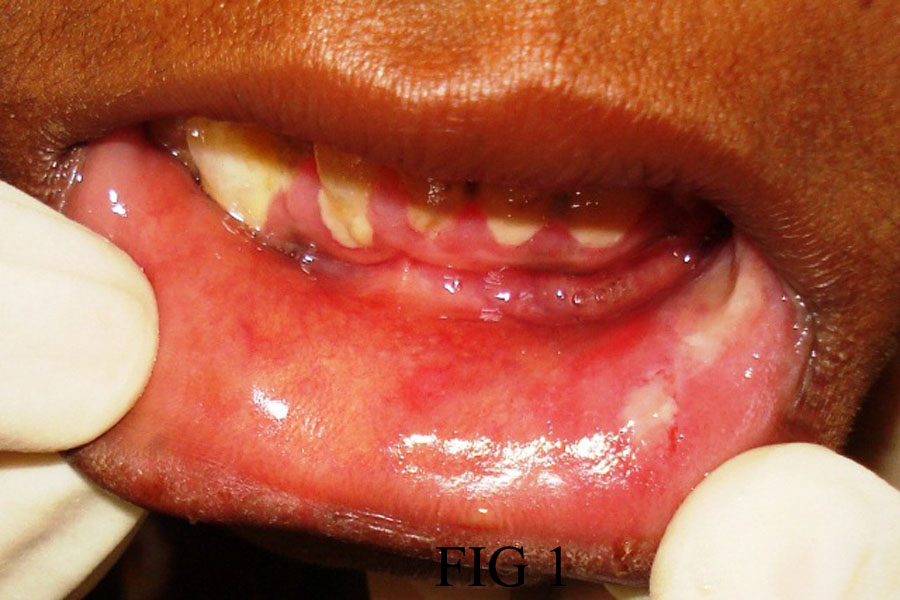
CASE 1: A 23-year-old female patient reported to the dental OPD with complaints of extensive painful ulcers and hemorrhagic crusts on the lips. She reported having painful oral ulceration and difficulty in eating. There is no significant family history except that she gave history of fever and common cold two weeks back for which she took azithromycin and diclofenac sodium, subsequently she developed irregular ulceration and hemorrhagic crust on the upper lip [Table/Fig-1]. On examination the upper lip was showing irregular ulcerations with yellow base and both right & left buccal mucosa showing irregular ulcerations [Table/Fig-2a,b]. Bilateral submandibular lymph nodes were enlarged and tender.
Irregular ulceration over lower lip
Irregular ulceration over right and left buccal mucosa
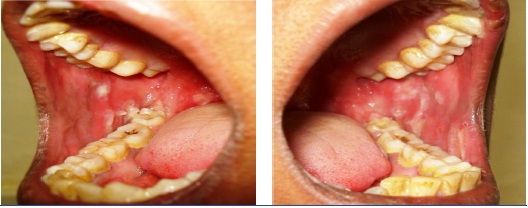
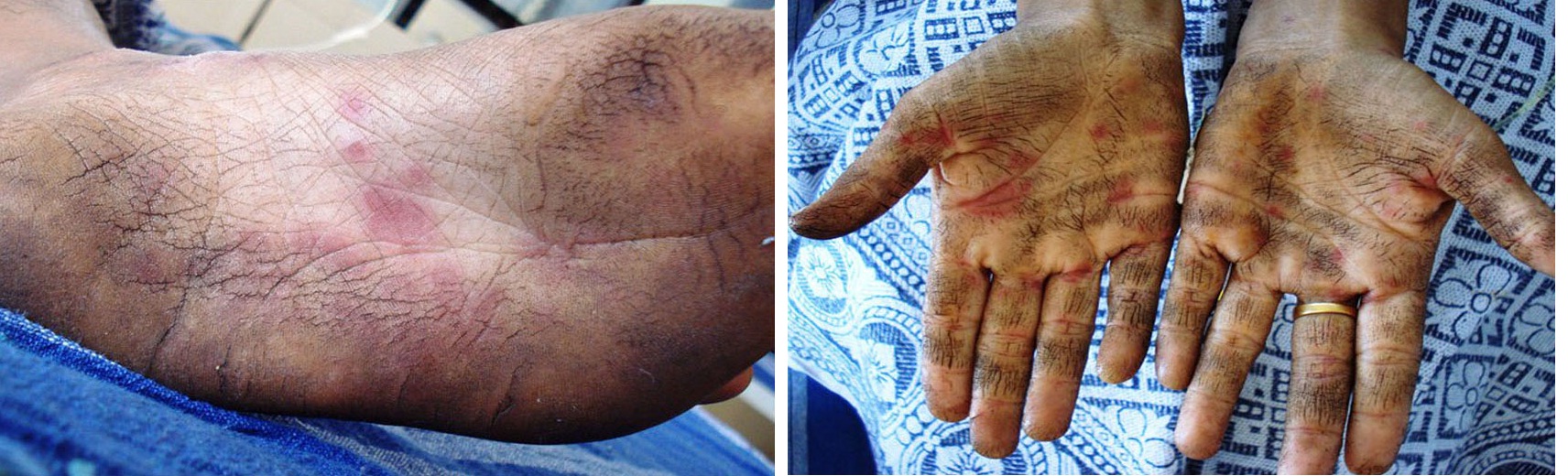
A diagnostically significant finding was presence of multiple typical target lesions on the dorsal and ventral surface of feet and erythematous lesions on the palmar and plantar surface of the hands [Table/Fig-3a,b,4a,b]. The patient was advised to discontinue the medication. Sudden onset of lesions, positive drug history associated with aggressive clinical features lead us to the diagnosis of EM.
Typical target lesion over skin the leg seen from frontal (A) and top view (B)
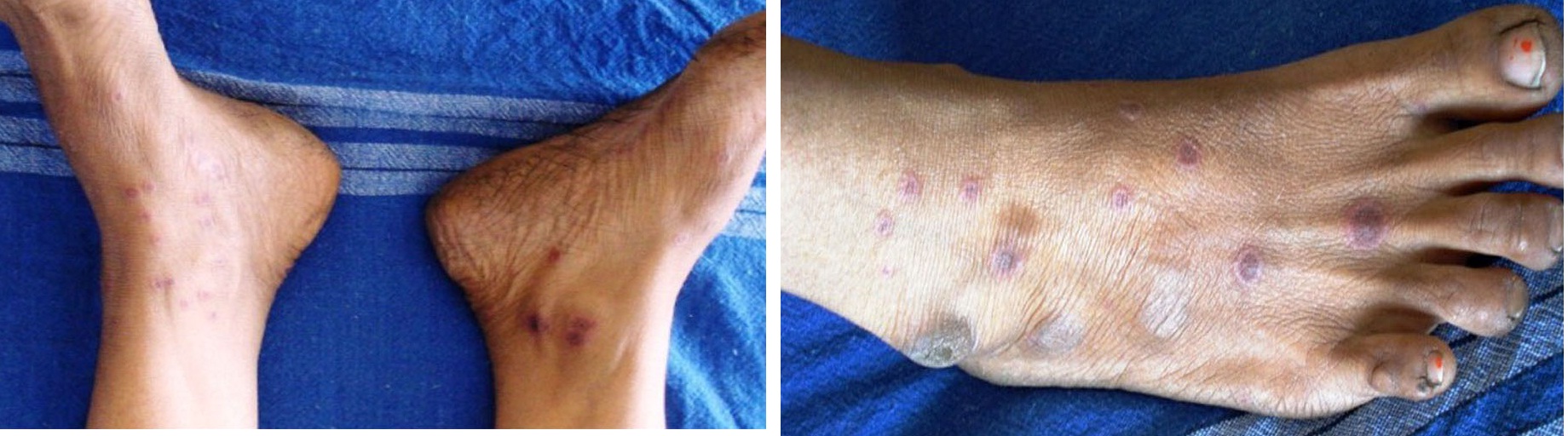
Classic target lesions over the sole of the foot (A) and on the palmar surface of hand as well (B)
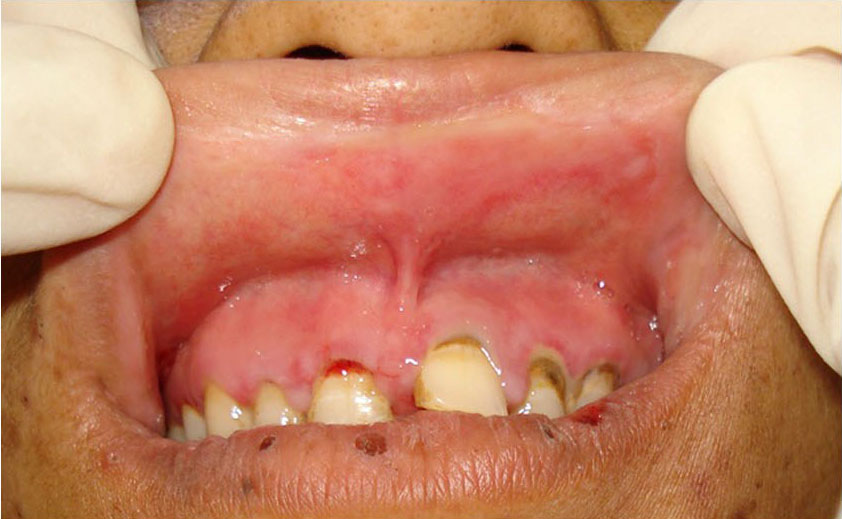
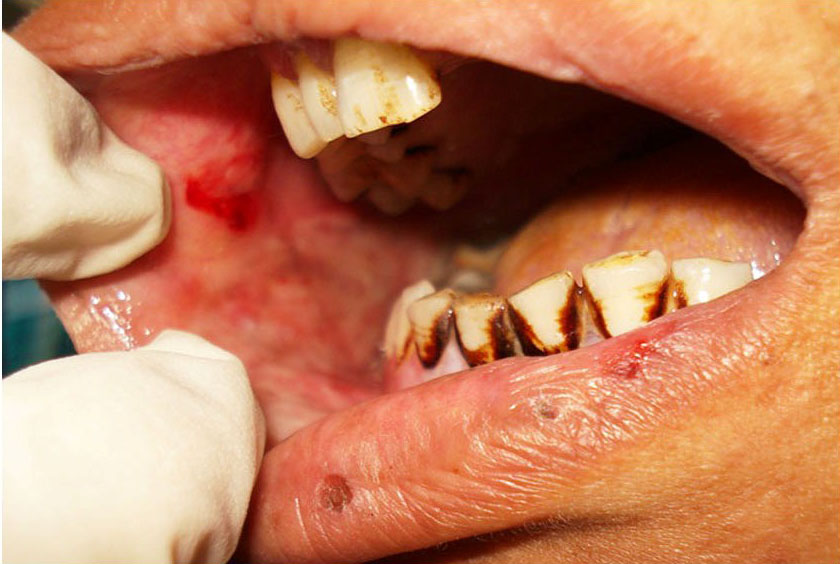
CASE 2: A 35-year-old female reported to the dental outpatient department with chief complaint of painful ulceration of the oral cavity for the past five days. Medical and family history was not significant. She gave history of swelling in relation with maxillary left central incisor for which she took amoxycilline & diclofenac Sodium few weeks back, within days she developed oral ulcerations. She gave a history of multiple vesicles of the oral mucosa on buccal and labial mucosa which ruptured to form painful ulcerations. After two days she developed ulcerations of lips [Table/Fig-5,6]. Patient was unable to eat solid food and was on liquid diet for the last two days. She had stopped medicines after developing vesicles. An oral examination showed ulcerations over the upper and lower lip [Table/Fig-5,6]. Intra orally white coating over the tongue and palate, & ulcerations over the right buccal mucosa were noted [Table/Fig-7]. Routine hematological investigations were advised and found within normal range. ESR was 25 mm in first hour by Westergreen’s method. Investigations for hepatitis B and C, and HIV were negative.
Irregular ulceration over lower lip
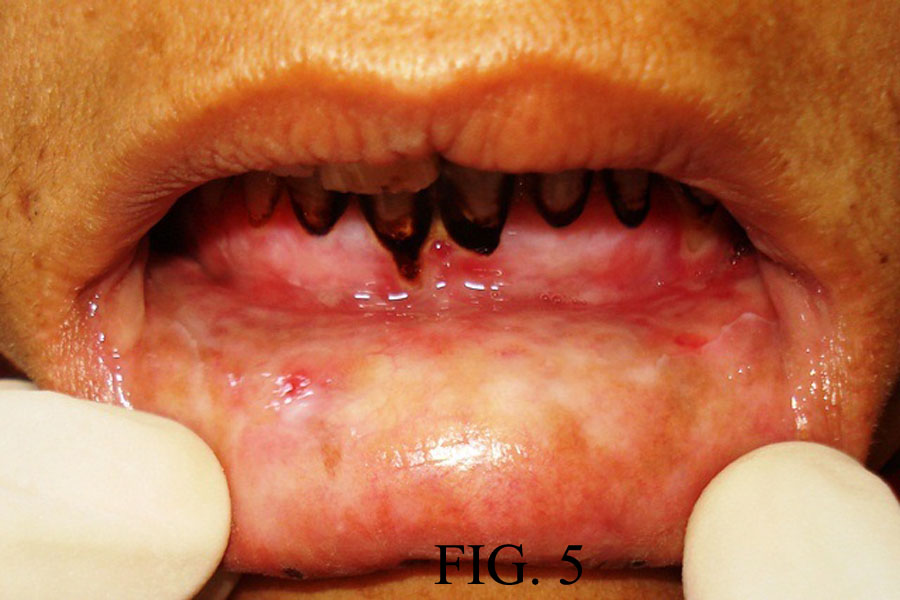
Irregular ulceration over upper lip
Irregular ulceration over right buccal mucosa
We were able to establish a temporal relationship between the drug intake and occurrence of the oral mucosal lesions. The oral ulcerations in our cases started within a few days of the drug intake and were resolved upon cessation of the drug. EM is usually triggered by herpes simplex infections, but rarely by drug intake.
Review of Literature
Adverse reaction to systemic drug administration can be manifested as EM, Steven Johnson syndrome, anaphylactic stomatitis, intraoral fixed drug eruptions, lichenoid drug reactions, and pemphigoid–like drug reactions [1]. It is manifested as skin eruption, with or without oral or other mucous membrane lesions [2–4]. It can be triggered by chemicals, drug intake or several infections [Table/Fig-8], in particular herpes simplex virus (HSV) infection, [2] which has been identified in up to 70% of EM cases [5]. Based on severity and number of mucosal sites involved, EM has been classified into EM Major and EM minor [6]. Oral EM shows typical mucosal ulcerations without any skin lesions. It has been reported that even if the primary attacks of oral EM are confined to the oral mucosa the subsequent attacks can produce more severe forms of EM involving the skin and hence it is important to identify and distinguish them from other ulcerative disorders involving oral cavity for early management and proper follow-up [7–10].
Causes of erythema multiformae [9–11]
| Infections | Drugs | Others |
|---|
VIRALHerpes Simplex Virus 1 and 2 Cytomegalovirus Varicella-Zoster Virus Hepatitis Viruses Epstein-Barr Virus
BACTERIAL:Mycoplasma Pneumoniae Mycobacterium Streptococci
FUNGAL INFECTIONS: PARASITES | ANTIBACTERIALSulfonamides Penicillins Cephalosporins Quinolones
ANTI CONVULSANTSBarbiturates Hydantoins Analgesics
ANTIFUNGALS CANCER CHEMO THERAPEUTIC | FOOD ADDITIVES OR CHEMICALS- Benzoates - Nitrobenzene - Terpenes - Ethanol
IMMUNE AND OTHER CONDITIONS- Inflammatory bowel disease - Pregnancy - Sarcoidosis - Systemic lupus erythematous
|
History
EM was first recognized in 1817 by Bateman and Bulkley, in 1846, reported the first American case as “Herpes Iris.” Later, Hebra, [12] in 1866 described the morphologic features of the eruption under the term “erythema exsudativum multiforme” and caused due to of internal or systemic origin and not local in causation [13]. The association of EM with severe vesiculobullous oral mucosal lesions with a lack of cutaneous lesions has produced confusing and bizarre clinical pictures. Stevens and Johnson, [14] in 1922, reported EM with predominant involvement of the oral and conjunctival mucous membranes as “a new eruptive fever associated with stomatitis and ophthalmia.”
Aetiology and Pathogenesis
The aetiology of EM is unclear in most patients, but appears to be an immunological hypersensitivity reaction with the CD8+ T lymphocytes, in epithelium, inducing apoptosis of scattered keratinocytes and leading to satellite cell necrosis [6]. A range of exogenous factors triggers an immunologically related reaction which appears as a sub and intra-epithelial vesiculation. There may be a genetic predisposition to EM, with associations of recurrent EM with HLA-B15 (B62), HLA-B35, HLA-A33, HLA-DR53 and HLADQB1* 0301. HLA DQ3 has been proven to be especially related to recurrent EM and may be a helpful marker for distinguishing HAEM (herpes-associated EM) from other diseases with EM-like lesions. Patients with extensive mucosal involvement may have the rare HLA allele DQB1*0402 [15]. Thus viral infections appear to trigger EM minor or major but drug ingestion tends to trigger more severe SJS or Toxic Epidermal Necrolysis (TEN) [16].
Drug-associated EM lesions test positive for tumor necrosis factor α and not interferon-γ as in herpes associated EM lesions, suggesting a varying mechanism [17]. The pathogenesis of herpes-associated EM has been well studied and is consistent with a delayed-type hypersensitivity reaction [17,18]. HSV genes within DNA fragments are expressed on keratinocytes, leading to the recruitment of HSV-specific CD4+ TH1 cells (helper T cells involved in cell-mediated immunity). The CD4+ cells respond to viral antigens with production of interferon-γ, initiating an inflammatory cascade [17].
Clinical Presentations
EM is a self limiting disease that usually has mild or no prodromal symptoms [19]. Patients may experience itching and burning at the site of the eruption [20]. The individual lesions begin acutely as numerous sharply demarcated red or pink macules that then become papular [19,21] with crusting or blistering sometimes occurs in the center of the lesions. The characteristic “target” or “iris” lesion has a regular round shape with three concentric zones: a central dusky or darker red area, a paler pink or edematous zone, and a peripheral red ring. Some target lesions have only two zones, the dusky or darker red centre and a pink or lighter red border [19,6].
Target lesions may not be apparent until several days after the onset, when lesions of various morphology clinically are present, hence the name erythema “multiforme” [22]. The skin lesions of EM usually appear symmetrically on the distal extremities and progress proximally [23]. Lesions on the dorsal surfaces of the hands and extensor aspects of the extremities are most characteristic [21]. Palms and soles also may be involved [20] Mucosal lesions may occur but usually are limited to the oral cavity [6]. EM resolves spontaneously in three to five weeks without sequelae, but it may recur [17]. Clinical variants of EM described in [Table/Fig-9].
| Infections | Drugs |
|---|
| EM minor | Typical target lesions, raised atypical target lesions, minimal mucous membrane involvement and, when present, at only 1 site (most commonly the mouth). Oral lesions; mild to severe erythema, erosions and ulcers. Occasionally may affect only the oral mucosa. < 10% of the body surface area is affected. |
| EM major | Cutaneous lesions and at least 2 mucosal sites (typically oral mucosa) affected. < 10% of the body surface area involved. Symmetrically distributed typical target lesions or atypical, raised target lesions or both. Oral lesions usually widespread and severe. |
| Stevens-Johnson syndrome | Main difference from EM major is based on the typology and location of lesions and the presence of systemic symptoms. < 10% of the body surface area is involved. Primarily atypical flat target lesions and macules rather than classic target lesions. Generally widespread rather than involving only the acral areas. Multiple mucosal sites involved, with scarring of the mucosal lesions. Prodromal flu-like systemic symptoms also common. |
| Overlapping Stevens-Johnson syndrome and toxic epidermal necrolysis | No typical targets; flat atypical targets are present. Up to 10%–30% of the body surface area affected. Prodromal flu-like systemic symptoms common. |
| Toxic epidermal necrolysis | When spots are present, characterized by epidermal detachment of > 30% of the body surface and widespread purpuric macules or flat atypical targets. In the absence of spots, characterized by epidermal detachment > 10% of the body surface, large epidermal sheets and no macules or target lesions. |
| Drug related erythema multiforme | Typically affect the oral mucosa, the lips and bulbar conjunctivae. Initially bullae rupture to give rise to haemorrhagic pseudo membrane of the lips and wide spread superficial oral ulcarations. |
| Drug related Toxic epidermal necronecrolysis | Toxic epidermal necrolysis (Lyell syndrome) is clinically characterised by extensive mucocutaneous epidermolysis preceded by a macular or maculopapular exanthema and enanthema (Lyell, 1979; Rasmussen et al, 1989). Intraorally there is widespread painful blistering and ulceration of all mucosal mucosal surfaces. Toxic epidermolysis may be associated with antimicrobials (sulphonamides and thiacetazone), analgesics (phenazones). antiepileptics, allopurinol, chlormezanone, rifampicin, fluconazole and vancomycin. |
Differential Diagnosis
Various lesions are to be considered which are confined to the oral region are herpes, autoimmune vesiculobullous lesions such as pemphigus vulgaris or bullous pemphigoid and other patterns of drug reactions. Our cases did not have any gingival ulceration. Extensive irregular ulcerations in the lining non keratinized mucosa were seen in our case 1 and case 2 [Table/Fig-10].
| Disorder | Different from EM |
|---|
| Urticaria | Itching is more severe. Each lesion usually disappears within 24 hours. Dermographism rubrum occurs. |
| Systemic Lupus Erythematous | Systemic symptoms occur (renal, arthritic etc) Laboratory findings of antinuclear antibodies, etc. EM sometimes occur in SLE |
| Bullous Pemphigoid | Direct/Indirect immunofluorescence reveals antibodies against basement membrane. |
| Acute Herpetic stomatitis | Ulcers are smaller with regular borders. |
Other patterns of drug reactions like lichenoid drug reactions, pemphigoid-like drug reactions can be easily differentiated based on the clinical patterns as above mentioned. Anaphylactic stomatitis often shows urticarial skin reactions with other signs and symptoms of anaphylaxis which were absent in our cases. In mucosal fixed drug eruptions the lesions are confined to localized areas of oral mucosa but in our cases there were wide spread lesions affecting labial, buccal, palatal, and tongue mucosa along with lip involvement [1].
Laboratory Findings: Because of inflammation, C-Reactive protein (CRP) may be positive and the erythrocyte sedimentation rate is elevated. The herpes simplex virus antibody titer, Mycoplasma antibody titer and antistreptolysin O (ASO) titer may be elevated in some cases. In cases involving bacterial infection, there is an increase in neutrophils [12]. The diagnosis is usually supported by peri-lesional tissue biopsy and exclusion of other causes.
Histopathology: Biopsy is advised in early vesicular lesions of EM not in ulcerated ones since histopathologic appearances are nonspecific and non-diagnostic [7].
In the early stages of epidermal EM, there is lymphocytic infiltration into the dermo-epidermal junction and vacuolar degeneration of basal cells. As the disease progresses, lymphocytes (CD8+T cells) infiltrate into the epidermis and necrosis of epidermal cells and subepidermal blistering are found. Histologically it is classified into three i.e epidermal, dermal and mixed [Table/Fig-11] [12]. Histological examination and immunostaining often shows moderate to dense perivascular inflammatory infiltrate (CD4+ Lymphocytes and histocytes) within the papillary dermis and along the dermoepidermal junction, dermal edema, intraepithelial/subepithelial vesicles and/or bullae, hydropic degeneration of basal keratinocytes and non-specific immune deposits of IgM, C3 and fibrin along basement membrane [6,24] The detection of intralesional HSV-DNA via polymerase chain reaction, as well as immunohistochemistry for IFN-c and TNF-a, may be useful tests to differentiate herpes associated EM from drug-associated EM [17].
Histopathological classification
| Classification | Main Histological Findings |
|---|
| Epidermal | In the early stage, lymphocytic infiltration and ballooning degeneration in the dermo-epidermal junction. As the disease progresses, infiltration of CD8+ lymphocytes into the epidermis, resulting in keratinocyte necrosis and subepidermal blistering. Decrease of epidermal Langerhans cells and overexpression of ICAM-1 on keratinocytes. |
| Dermal | Perivascular monocytic infiltration in the upper dermis; edema in the dermal papilla. It is now said that EM is always accompanied by at least some change in the epidermis. |
| Mixed | Epidermal changes (vacuolar degeneration of the basal layer, satellite cell necrosis); dermal changes (perivascular lymphocytic infiltration). |
Treatment: Despite the advancement in the diagnosis, still no single specific treatment modality is available. There should be identification and withdrawl of the causative agent/drug along with supportive care. Mild cases of oral EM are treated mainly with sympathetic measures including application of topical anesthetic mouthwashes and liquid and soft diet. Moderate to severe cases of oral EM can be treated with a short course of systemic corticosteroids in patients without any significant contraindications to their use. Systemic corticosteroids should only be used by clinicians familiar with the side effects, and, in each case, potential benefits should be carefully weighed and the dose should be tapered over 2 to 3 weeks. Recently immunomodulating/immunosuppressive drugs (Dapsone, Azathioprine, Levamisole) are showing promising results in suppression of disease progression [24].
For CASE 1- Oral corticosteroid (methylprednisolone at the dose of 32 mg/ day) was started. Within 5 days, all the mucosal lesions healed and methylprednisolone was stopped after tapering over next 7 days. Mouthwashes consisting of local anaesthetics and antiseptics were added to aid in oral fluid intake.
For CASE 2- Patient was treated with corticosteroid twice a day for 3 days followed by tapering dose for 10 days and local application of topical anesthetic gel for pain relief.
The lesions completely regressed after 10 to 12 days in both cases.
Conclusion
Drug induced Oral EM is a rare and less described variant of Erythema Multiformae. EM is often triggered by HSV infections and rarely by adverse drug reactions. Even though primary attack of drug induced EM is confined to the oral mucosa the subsequent attack can produce more severe forms of EM (EM minor, EM major) involving their skin. It is important for oral pathologists and general dentists to differentiate from other vesicullobullous lesions from drug induced EM for prompt management and proper follow-up.
[1]. Neville BW, Damm D, Allan CM, Allergic and immunologic diseasesIn: Oral and Maxillofacial Pathology 2002 2nd edPhiladelphiaSaunders:285-314. [Google Scholar]
[2]. Bastuji-Garin S, Rzany B, Stern RS, Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and EMArch Dermatol 1993 129:92-96. [Google Scholar]
[3]. Al-Johani KA, Fedele S, Porter SR, EM and related disordersOral Surg Oral Med Oral Pathol Oral Radiol Endod 2007 103(5):642-54. [Google Scholar]
[4]. Farthing P, Bagan JV, Scully C, Mucosal diseases series. Number IV. EMOral Dis 2005 11(5):261-67. [Google Scholar]
[5]. Ayangco L, Rogers RS 3rd, Oral manifestations of EMDermatol Clin 2003 21:195-205. [Google Scholar]
[6]. Kennett S, EM affecting the oral cavityOral Surg Oral Med Oral Pathol 1968 25:366-73. [Google Scholar]
[7]. Bean SF, Quezada RK, Recurrent oral EM clinical experience with 11 patientsJAMA 1983 249:2810-12. [Google Scholar]
[8]. Osterne RL, Matos Brito RG, Pacheco IA, Management of EM associated with recurrent herpes infection: a case reportJ Can Dent Assoc 2009 75(8):597-601. [Google Scholar]
[9]. Lamoreux MR, Sternbach MR, Hsu WT, EMAm Fam Physician 2006 74(11):1883-88. [Google Scholar]
[10]. Kohli PS, Kaur J, EM-oral variant: case report and review of literatureIndian J Otolaryngol Head Neck Surg 2011 63(Suppl 1):9-12. [Google Scholar]
[11]. Hiroshi Shimizu, Erythema, Erythroderma (Exfoliative Dermatitis)In Shimizu’s Textbook of Dermatology 2007 :115-20. [Google Scholar]
[12]. Hebra F, Diseases of the skin. Fagge CH (Ed) 1866 Vol. ILondonNew Sydenham Society [Google Scholar]
[13]. Stevens AM, Johnson FC, A new eruptive fever associated with stomatitis and ophthalmiaAm J Dis Child 1922 24:526-33. [Google Scholar]
[14]. Farthing P, Bagan JV, Scully C, Mucosal disease series. Number IV. EMOral Dis 2005 11(5):261-67. [Google Scholar]
[15]. Auquier-Dunant A, Mockenhaupt M, Naldi L, SCAR Study Group. Severe Cutaneous Adverse Reactions. Correlations between clinical patterns and causes of EM majus, Stevens-Johnson syndrome, and toxic epidermal necrolysis: results of an international prospective studyArch Dermatol 2002 138(8):1019-24. [Google Scholar]
[16]. Aurelian L, Ono F, Burnett J, Herpes simplex virus (HSV)-associated EM (HAEM): a viral disease with an autoimmune componentDermatol Online J 2003 9(1):1 [Google Scholar]
[17]. Kokuba H, Aurelian L, Burnett J, Herpes simplex virus associated EM (HAEM) is mechanistically distinct from drug-induced EM: interferon-gamma is expressed in HAEM lesions and tumor necrosis factor-alpha in drug-induced EM lesionsJ Invest Dermatol 1999 113(5):808-15. [Google Scholar]
[18]. Odom RB, James WD, Berger TG 21, Erythema and urticariaIn:Andrew’s diseases of the skin: clinical dermatology 2000 9th edPhiladelphia, PAW.B. Saunders Co:95-146-51. [Google Scholar]
[19]. Habif TP, Hypersensitivity syndromes and vasculitisIn: Clinical Dermatology: A Color Guide to Diagnosis and Therapy 2004 4th edNew YorkMosby:626-34. [Google Scholar]
[20]. Shin HT, Chang MW, Drug eruptions in childrenCurr Probl Pediatr 2001 31:207-34. [Google Scholar]
[21]. Huff JC, EM and latent herpes simplex infectionSemin Dermatol 1992 11:207-10. [Google Scholar]
[22]. Volcheck GW, Clinical evaluation and management of drug hypersensitivityImmunol Allergy Clin North Am 2004 24(3):357-71. [Google Scholar]
[23]. Howland WW, Golitz LE, Weston WL, Huff JC, EM: clinical, histopathologic, and immunologic studyJ Am Acad Dermatol 1984 Mar 10(3):438-46. [Google Scholar]
[24]. Greenberg MS, Glick M, Ship JA, Burket’s Oral medicine 2008 Eleventh editionBC Decker:57-60. [Google Scholar]