Background: Lymph nodes undergo reactive changes in response to a wide variety of stimuli, the most common cause being inflammatory and immune reactions, apart from primary malignant neoplasms and metastatic tumours. It is well accepted that a clinical examination alone cannot be considered diagnostic to justify the involvement of cervicofacial lymph nodes especially deep and small nodes. Ultrasonography is an easy, reproducible, non invasive, non-ionizing imaging modality to evaluate cervicofacial lymph nodes.
Aim: The present study was devised with an aim of comparing the clinical and ultrasonographic features of cervicofacial lymphadenopathy.
Materials and Methods: The subjects for the study were selected from the patients who visited the outpatient department with clinically palpable lymph nodes. Fifty two patients were included in the study and they were divided into 4 groups; group I (subjects with odontogenic infections), group II (subjects with non odontogenic oral conditions), group III (subjects with tuberculosis) and group IV (subjects with head and neck carcinomas). A detailed case history was recorded and a thorough clinical examination was carried out for all subjects. The cervicofacial lymph nodes were palpated and examined. Ultrasonographic examination of cervicofacial lymph nodes was carried out and recorded. The statistical analysis was done using chi square test.
Result and Conclusion: Most of the subjects considered for the study showed cervicofacial lymphadenopathy associated with odontogenic causes, specific infections like tuberculosis and head and neck malignancies. The lymph nodes showed varied clinical features. The ultrasonographic features such as number, size, shape, short axis/long axis ratio, border sharpness, hilum, echogenicity, distribution of the internal echoes, intranodal necrosis, matting or soft tissue edema were evaluated. The results were tabulated and statistical analysis was done.
Introduction
Lymph nodes undergo reactive changes in response to a wide variety of stimuli which include microbial infections, drugs, environmental pollutants, tissue injury, immune complexes and neoplasia. However, the most common cause of lymph node enlargement are inflammatory and immune reactions, apart from primary malignant neoplasms and metastatic tumours. Cervicofacial lymphadenopathy is a common finding in infections like odontogenic infections, tuberculosis and in head and neck malignancies. It is well accepted that clinical examination alone cannot be considered as diagnostic to justify the involvement of cervicofacial lymph nodes especially the deep or small nodes. Assessment of cervicofacial lymph nodes is essential and important for patients with head and neck malignancies as it helps in treatment planning and prediction of prognosis. Hence, investigations such as ultrasonography will prove as an adjunctive diagnostic tool in the evaluation of cervicofacial lymph nodes.
Ultrasonography is a useful imaging modality in the assessment of cervicofacial lymph nodes. High-resolution ultrasonography is a useful imaging tool for the assessment of cervicofacial lymph nodes because of its high image resolution. Therefore, it is suitable for both monitoring disease progression and follow-up assessment after treatment. Ultrasonography is an easy, reproducible, non-invasive, no risk procedure, radiation free imaging modality to evaluate cervicofacial lymph nodes. The gray scale sonographic features that help to distinguish the various features of cervicofacial lymphadenopathy include the size, shape, short axis/long axis ratio, border unsharpness, hilum, echogenicity, distribution of the internal echoes, the presence of intranodal necrosis and calcification [1,2].
In the evaluation of cervicofacial lymph nodes ultrasonography is considered to be superior to other imaging modalities such as computed tomography and magnetic resonance imaging, as computed tomography is less sensitive than ultrasonography in the detection of small nodes [3]. Magnetic Resonance Imaging is expensive, not as easily available as ultrasonography and is also not suitable for patients with pacemakers or with claustrophobia[1].
Aim of the Study
The present study was devised with an aim of comparing the clinical and ultrasonographic features of cervicofacial lymphadenopathy seen in subjects visiting the dental outpatient department of Oral Medicine and Radiology.
Objectives of the Study
1. To evaluate the clinical features in different forms of cervical lymph nodes.
2. To detect, delineate and evaluate features of cervicofacial lymph nodes using ultrasonography.
3. To compare clinical and ultrasonographic features of the cervicofacial lymph nodes in different forms of lymphadenopathy.
Materials and Methods
The subjects for the present study were selected from the outpatient department of Oral Medicine and Radiology, D.A.P.M.R.V Dental College, Bangalore, India between July 2010 and February 2011. Fifty two subjects with clinically palpable lymph nodes were included in the study. The subjects included in the study were above 18y of age. An informed consent was obtained from all the subjects selected for the study. These subjects were further categorized into 4 subgroups:-
• Group I : Subjects with odontogenic infections- 30 subjects (41 nodes).
• Group II: Subjects with non odontogenic oral conditions- 11subjects ( 18 nodes)
• Group III: Subjects with Tuberculosis- 5 subjects (16 nodes)
• Group IV: Subjects with Head and neck carcinoma- 6 subjects ( 14 nodes)
A detailed case history including past medical history was recorded. The cervicofacial lymph nodes were palpated and examined under appropriate aseptic precautions. The following features such as location, size, shape, consistency, tenderness, mobility, drainage and matting was recorded. Ultrasonographic examination of cervicofacial lymph nodes was carried out on the same day using linear array transducer utilizing a frequency of 5-7 MHz. The following criteria were evaluated:- number, size, shape, short axis/long axis ratio, border sharpness, hilum, echogenicity, distribution of the internal echoes, intranodal necrosis, and matting or soft tissue oedema. The clinical features and ultrasonographic features were compared and the results were tabulated. The statistical analysis was done using chi square test.
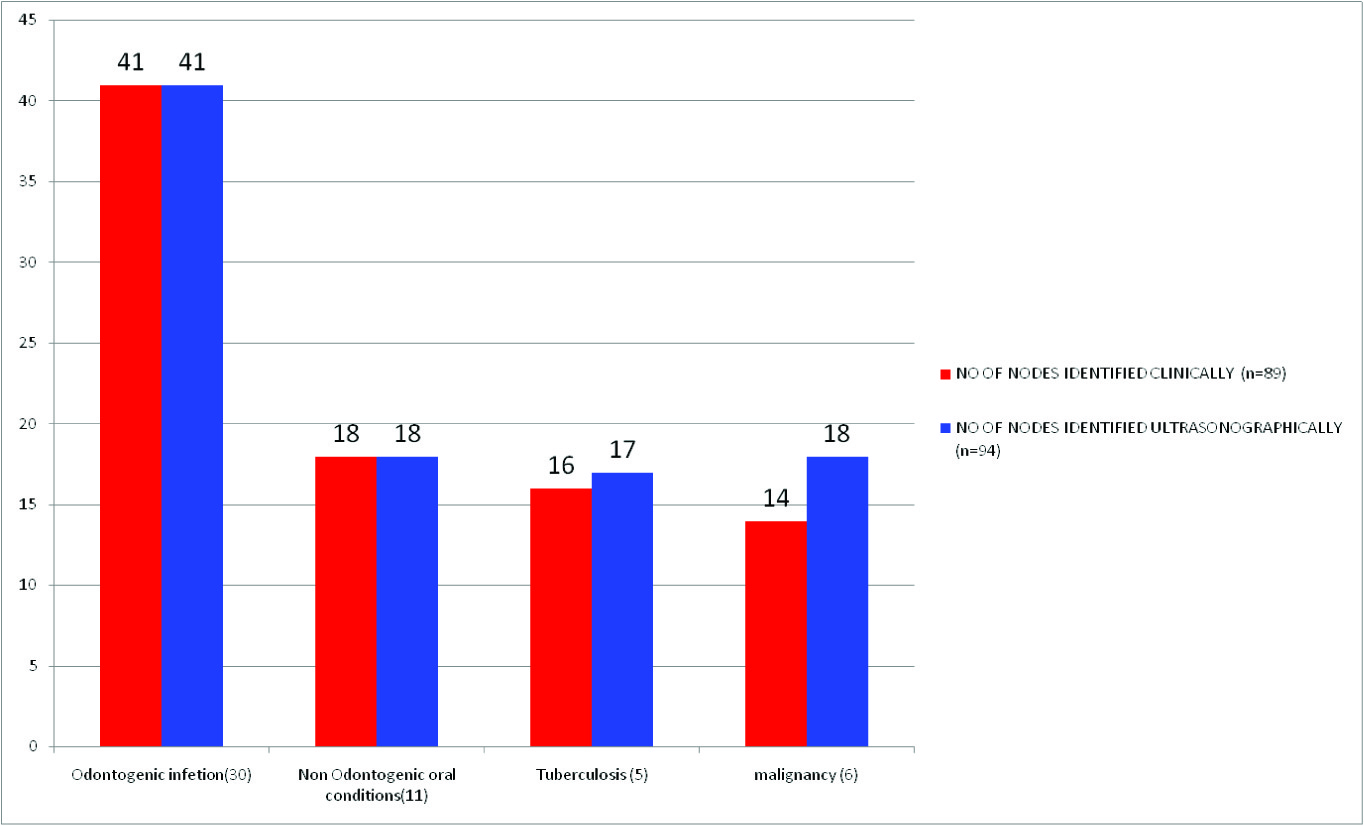
Distribution of lymph nodes detected on clinical < ultrasonographic Examination
| Category | consistency | tenderness | fixity |
| Firm | Hard | Tender | Non Tender | Movable | Fixed |
| Odotogenic (41) | 41 | 0 | 37 | 4 | 41 | 0 |
| Non odontogenic (18) | 18 | 0 | 15 | 3 | 18 | 0 |
| Tuberculosis (16) | 16 | 0 | 16 | 0 | 16 | 0 |
| Malignancy (14) | 7 | 7 | 10 | 4 | 7 | 7 |
| Total | 82 | 7 | 78 | 11 | 82 | 7 |
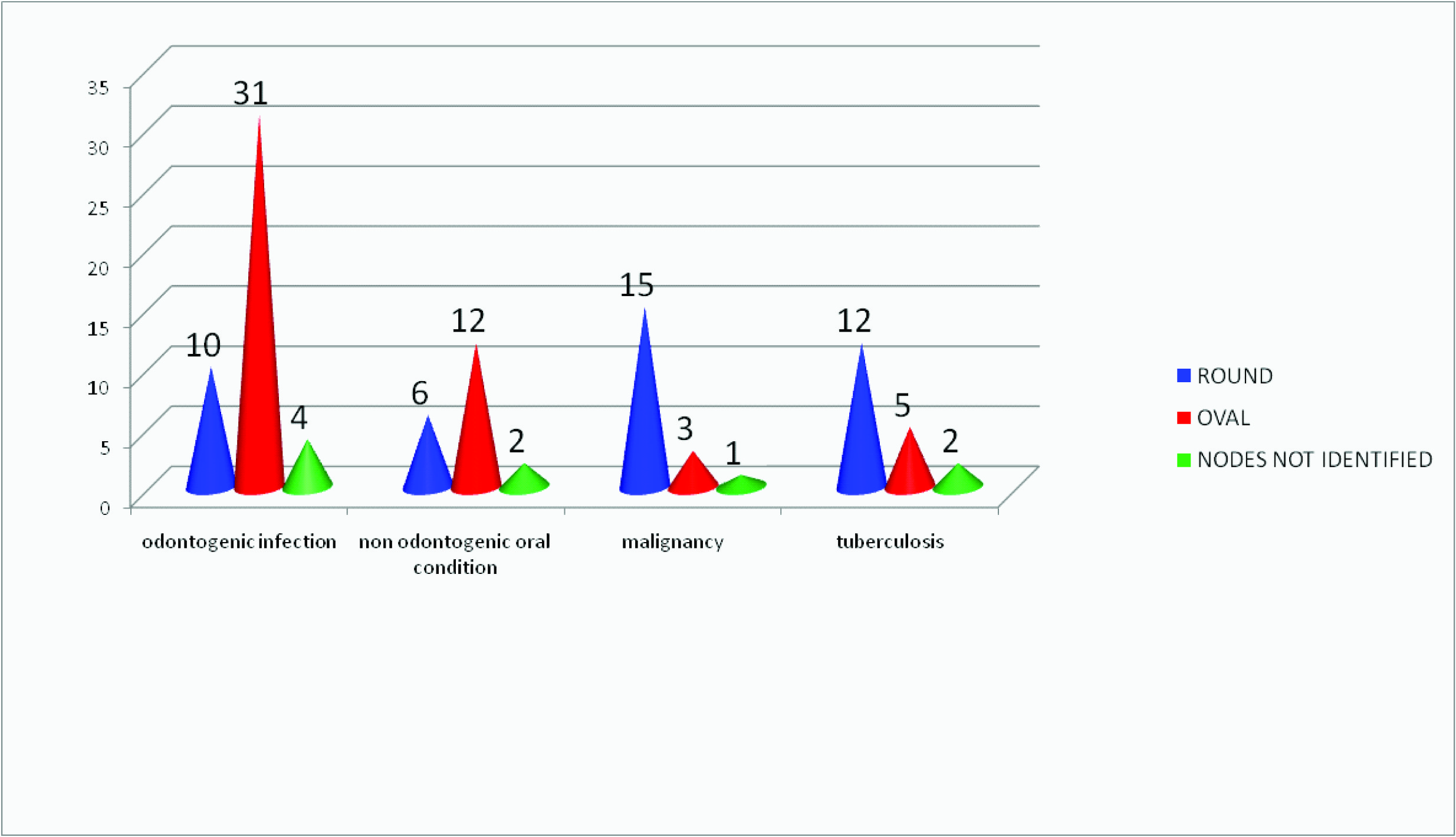
Shape of nodes observed in the study subjects
Size of the nodes in the study subject groups based on s/l ratio
| Category | Number | Smallest Node (mm) | Largest Node (mm) |
| Odontogenic | 41 | 7x5 | 20x12 |
| Non odontogenic | 18 | 9x4 | 24x9 |
| Tuberculosis | 17 | 9x6 | 24x12 |
| Malignancy | 18 | 8x7 | 40x20 |
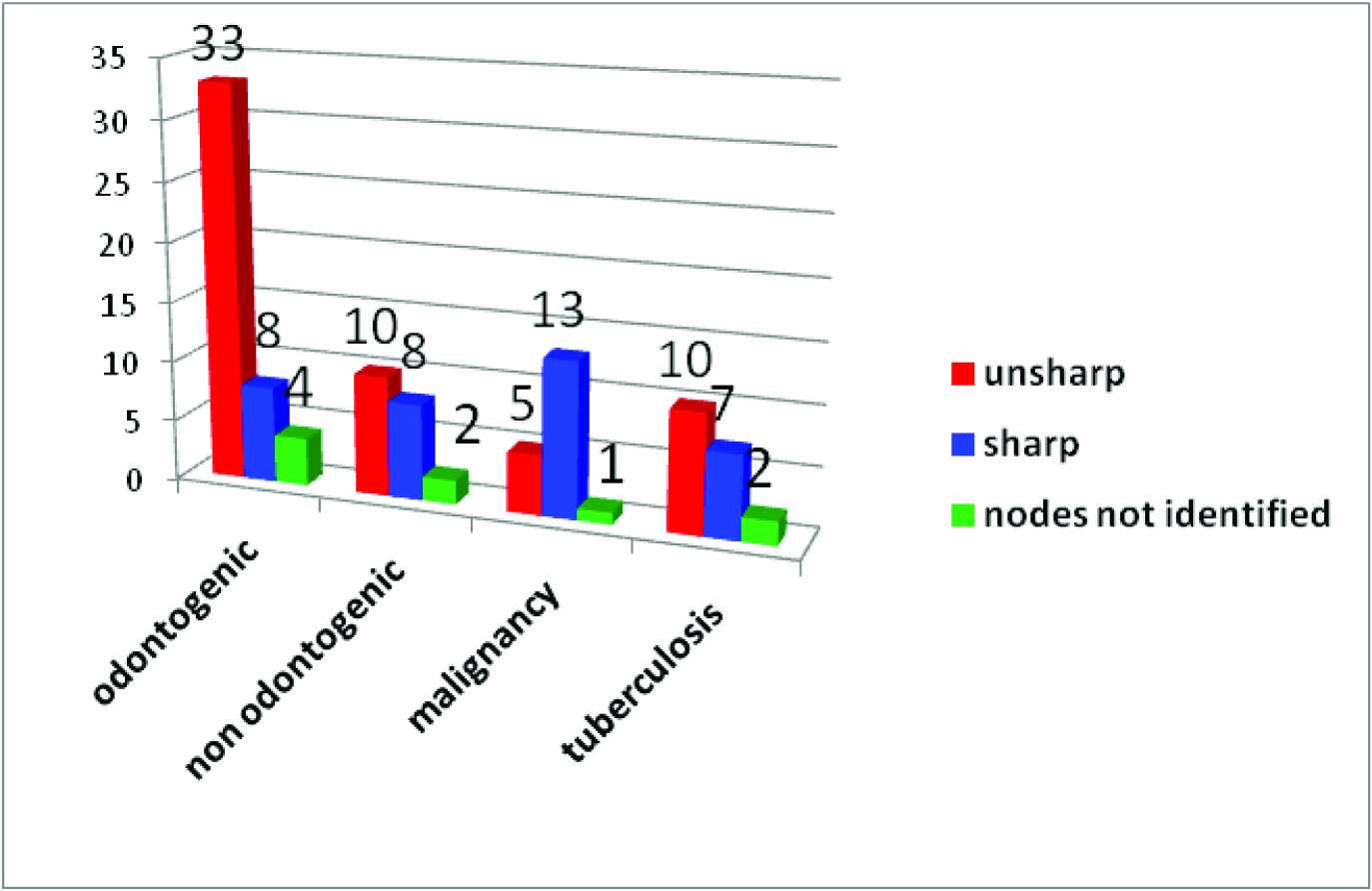
Border sharpness observed in 94 nodes
Presence or absence of hilum observed in 94 nodes
Echogenecity observed In 94 Nodes
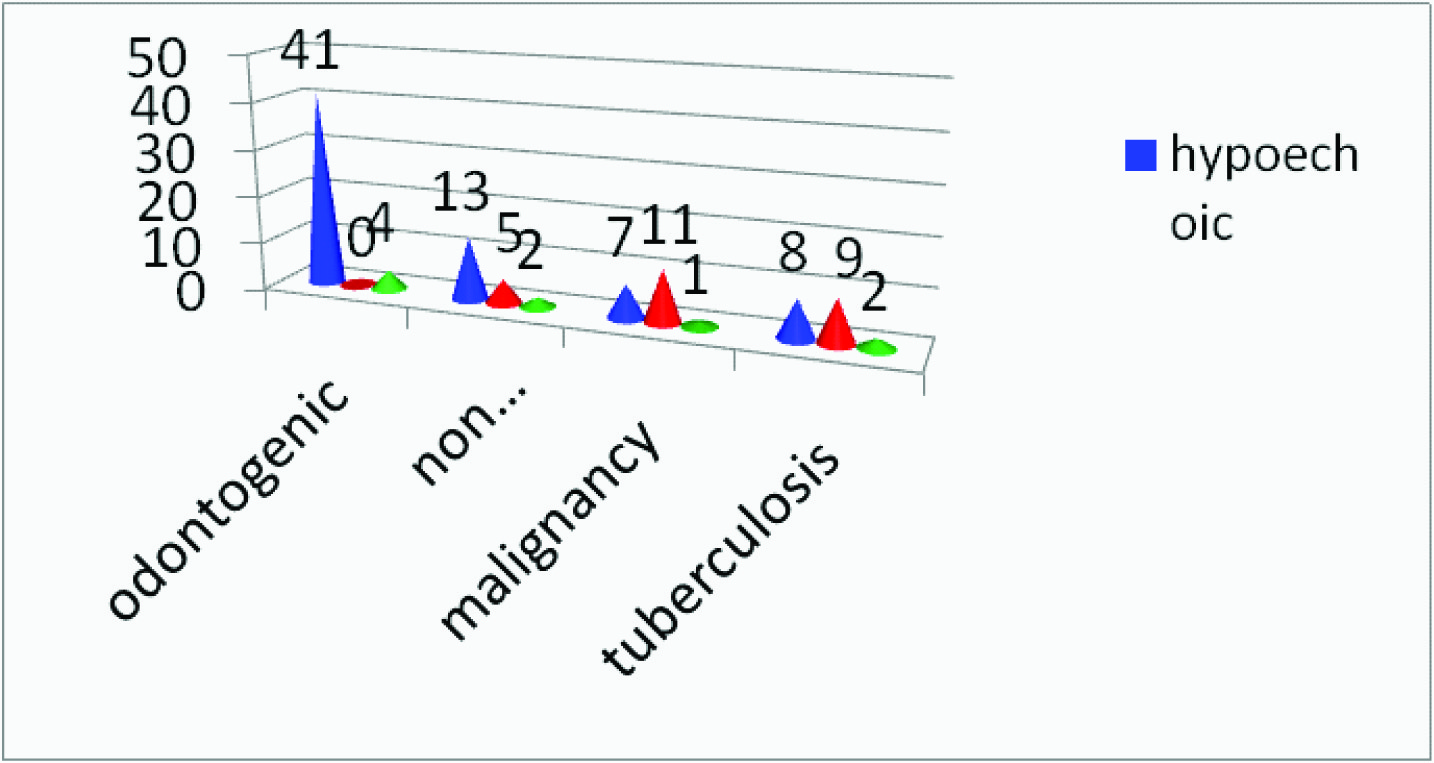
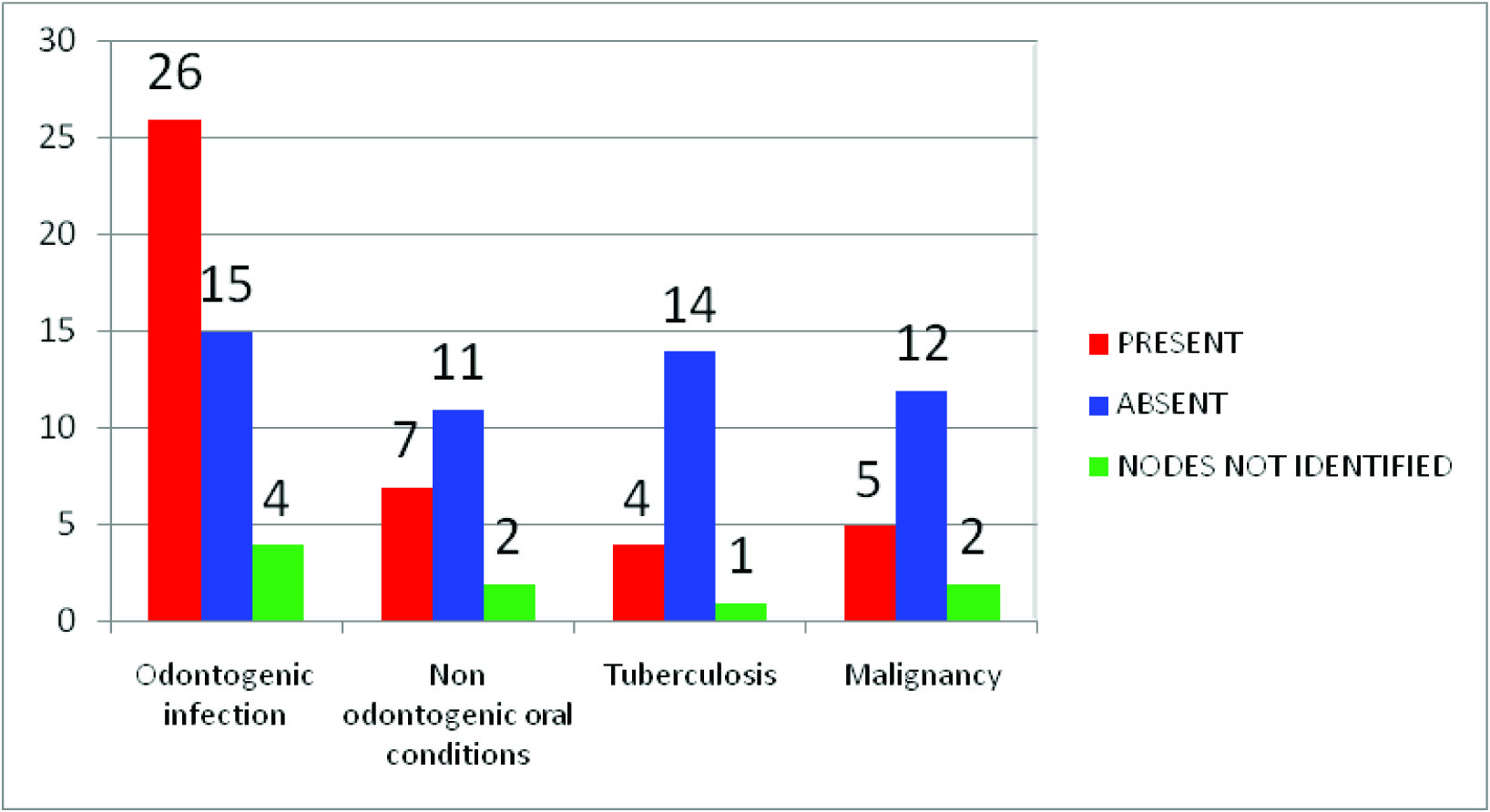
Presence or absence of necrosis observed in 94 nodes
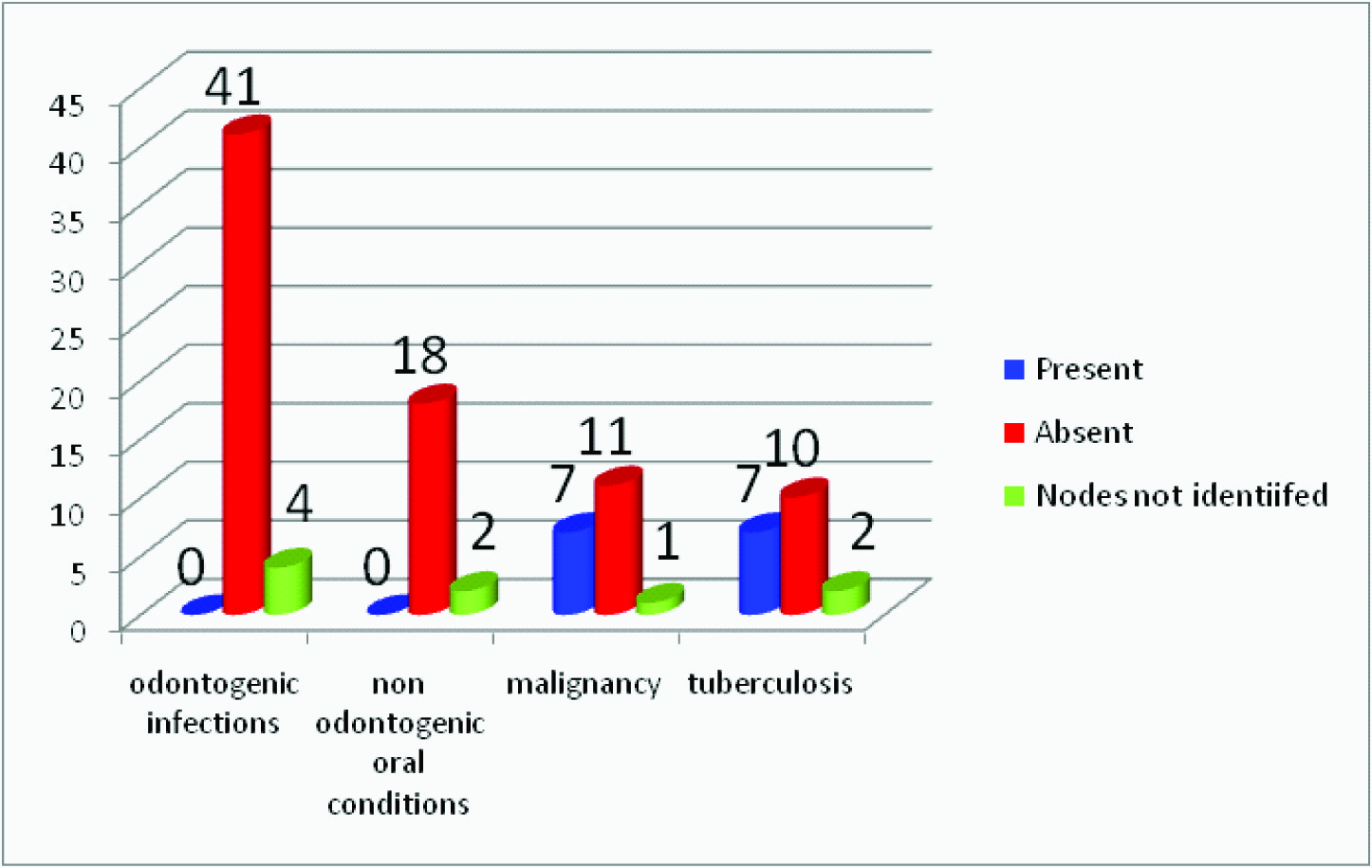
Prescence or absence of matting observed in 94 nodes
Results
Among the 52 subjects, 89 lymph nodes were identified by clinical examination and 94 lymph nodes were identified by ultrasonographic examination [Table/Fig-1]. The causes of cervicofacial lymphaden- opathy in odontogenic infections (Group I) were dental caries (34%), chronic generalized gingivitis/periodontitis (CGG/CGP) (23%), abscesses associated with odontogenic infections (16%), pericoronitis (15%) and apical periodontitis (12%).
The causes of cervicofacial lymphadenopathy in non odontogenic oral conditions (Group II) were oral submucous fibrosis (29%), oral ulcers (24%), lichenoid reaction/ lichen planus (14%) and others (33%) which included lipoma, pyogenic granuloma, sialadenitis etc.
Clinical examination of the lymph nodes in the study subjects with odontogenic infection showed that all the lymph nodes were firm, movable and 90% nodes were tender on palpation. All the lymph nodes associated with non odontogenic oral conditions were also firm, movable and 83% of the lymph nodes were tender. All the lymph nodes associated with tuberculosis were movable and tender. Fifty percent of the lymph nodes associated with malignancy were fixed, stony hard on palpation and 71% of the lymph nodes were tender on palpation [Table/Fig-2].
Ultrasonographic features of cervicofacial lymphnodes
On ultrasonographic examination the shape of the lymph nodes showed significant difference between the four groups namely odontogenic and non odontogenic inflammatory lymph nodes, tuberculous lymph nodes and malignant lymph nodes with p-value <0.001. Border sharpness also showed significant difference between the groups with p-value of 0.014. Echogenicity, internal echoes, matting and necrosis showed significant difference between the four groups with p-value <0.001. The presence or absence of echogenic hilum did not show any statistically significant difference between the groups with p-value of 0.445.
Discussion
In the present study it was observed that ultrasonographic features like shape, border sharpness, echogenicity, presence or absence of matting, intra nodal necrosis were significant in differentiating malignant and tuberculous lymph nodes from inflammatory lymph nodes.
Normal and reactive lymph nodes are usually oval in shape where as malignant lymph nodes and tuberculous lymph nodes tend to be round [1]. Although pathologic lymph nodes are usually round occasionally normal submandibular and parotid lymph nodes can also be round in shape [1]. Therefore, the shape of lymph nodes cannot be a sole criterion in the ultrasonographic diagnosis. In the present study however the shape of the lymph nodes showed significant variation depending on the cause, as majority of the normal and reactive lymph nodes were oval and malignant and tuberculous lymph nodes were round in shape [Table/Fig-3].
The ‘size’ of the lymph nodes was measured along two axes: the short axis (S) and the long axis (L) and noted in millimeters. Size has been used to differentiate reactive lymph nodes from malignant lymph nodes [2]. Normal cervical lymph nodes have an S/L ratio less than 0.5 and the tuberculous and metastatic lymph nodes have S/L ratio greater than 0.5 [1]. This indicates that large size lymph nodes have a higher potential of being malignant, however occasionally reactive lymph nodes can also be as large as malignant lymph nodes [1]. In the present study the mean size of the lymph nodes with odontogenic infection on S/L ratio were 0.47, lymph nodes associated with non odontogenic oral conditions were 270.54, tuberculous lymph nodes were 0.61 and malignant lymph nodes were 0.69 [Table/Fig-4] . Different cut–off values of nodal size ranging from 5mm to 10mm have been reported. As the cut-off point of nodal size increases, the sensitivity of the size criteria in differentiating reactive lymph node from malignant lymph node decreases while the specificity increases[3]. Nodal size is useful in clinical practice, in subjects with known malignancy. When the size of lymph nodes increases on serial ultrasonographic examination it is said to be highly suspicious for metastasis. A progressive change of nodal size is also useful to monitor the treatment response of the subjects with malignancy [1].
Normal lymph nodes have unsharp borders [4]. This is related to the associated oedema and inflammation of surrounding soft tissues. Malignant lymph nodes on the other hand tend to have sharp borders, due to the fact that tumour infiltration causes an increase in the difference of acoustic impedance between intra nodal region and surrounding tissues [4,5]. An unsharp border may be found in tuberculosis lymph nodes, and again this is related to the associated oedema and inflammation of the surrounding soft tissues [1,5]. Malignant lymph nodes in advanced stages may also show an ill- defined border, indicating extracapsular spread and this has shown to reduce the survival rate of the subjects by 50% [1].
In the present study majority of the odontogenic and non odontogenic inflammatory lymph nodes and lymph nodes associated with tuberculosis had unsharp borders and only the lymph nodes associated with malignancy had sharp borders. A statistically significant difference existed in border sharpness between lymph nodes associated with malignancy, tuberculosis and odontogenic and non odontogenic inflammatory lymph nodes [Table/Fig-5].
The echogenic hilus is mainly the result of multiple medullary sinuses, each of which acts as an acoustic interface, which partially reflects the ultrasound waves and produces an echogenic structure. Fatty infiltration makes the hilus more obvious in ultrasononography [6]. On ultrasonographic examination the echogenic hilus appears as an hyperechoic linear structure and is continuous with the adjacent fat [1]. Neck lymph node with a maximum transverse diameter greater than 5mm shows an echogenic hilus [1]. The incidence of echogenic hilus increases with age which is probably related to the increased fatty deposition in the lymph nodes of elderly individuals [1,7].
In the present study majority of the inflammatory lymph nodes showed echogenic hilum where as in majority of the lymph nodes associated with malignancy and tuberculosis the hilum was absent [Table/Fig-6]. The presence of echogenic hilus within the lymph nodes is considered as a sign of benignity. Malignant lymph nodes usually do not show echogenic hilus. However, it has been reported that echogenic hilus may also be found in malignant lymph nodes and small normal nodes [6,8]. Therefore, the presence or absence of echogenic hilus should not be used as a sole criterion for evaluating cervicofacial lymphadenopathy.
Malignant lymph nodes are predominantly hypoechoic when compared to adjacent soft tissues except in case of metastatic lymph nodes of papillary carcinoma of thyroid which are commonly hyperechoic [9]. In malignant diseases, the process involves infiltration of the nodes by malignant cells which is more likely to result in early distortion of internal nodal architecture showing as heterogeneity on ultrasound [10,11]. Tuberculous lymph nodes tend to be hypoechoic which is related to intranodal cystic necrosis. In the present study most of the inflammatory lymph nodes were hypoechoic and the lymph nodes associated with malignancy and tuberculosis showed mixed echotexture. A statistical significant difference existed in the echogenicity between lymph nodes associated with malignancy, tuberculosis and inflammatory lymph nodes [10,11] [Table/Fig-7] .
Lymph nodes with intranodal necrosis, regardless of their size are pathologic [7]. Necrosis may manifest itself as a true cystic area within the lymph node (cystic necrosis) or present as an area of hyperechogenicity within a lymph node (coagulation necrosis)[1]. The present study showed necrosis in the lymph nodes of seven subjects with tuberculosis and seven subjects with malignancy where as the other groups showed no necrosis, thus making intranodal necrosis a significant feature in differentiating between lymph nodes associated with malignancy, tuberculosis and inflammatory lymph nodes [Table/Fig-8] .
Matting is considered when there are clumps of multiple abnormal lymph nodes with no normal intervening soft tissues. It is a common feature in tuberculous lymphadenitis as a result of periadenitis and adjacent soft tissue oedema [12,13]. It is a useful feature to differentiate tuberculosis from other diseases. In the present study matting was absent in the lymph nodes associated with odontogenic and non odontogenic causes whereas majority of the tuberculous lymph nodes showed matting [Table/Fig-9] .
A recent study demonstrated that triplex ultrasonographic characterization of cervical lymph nodes could differentiate malignant from benign nodes with an acceptable degree of certainty [14].
Conclusion
Ultrasonographic evaluation is more sensitive than clinical examination in the detection of the cervicofacial lymph nodes as well as in differentiating malignant and tuberculous lymph nodes from inflammatory lymph nodes. Ultrasonographic evaluation when supplemented with clinical examination of cervicofacial lymph nodes ay help in over all preoperative treatment planning, management, there by contributing in early diagnosis and a better prognosis of patients which inturn helps to improve the quality of life of these patients.
[1]. Ying M, Pang BSF, Three-dimensional ultrasound measurement of cervical lymph node volume. The British Journal of Radiology. 2009 82:617-25. [Google Scholar]
[2]. Ahuja Anil T, Ying Michael, Sonographic Evaluation of Cervical Lymph Nodes AmericanJournal of Roentgenology 2005 184:1691-99. [Google Scholar]
[3]. M Ying, A Ahuja, C Metreweli, Diagnostic accuracy of sonographic criteria for evaluation of cervical lymphadenopathy.J Ultrasound Med. 1998 17:437-45. [Google Scholar]
[4]. M Shozushima, M Suzuki, T Nakasima, Y Yanagisawa, K Sakamaki, Y Takeda, Ultrasound diagnosis of lymph node metastasis in head and neck cancer.Dentomaxillofac Radiol. 1990 19:165-70. [Google Scholar]
[5]. A Pandey, SN Kureel, J Pandey, A Wakhlu, J Rawat, TB Singh, Chronic cervical lymphadenopathy in children: Role of ultrasonography.J Indian Assoc Pediatr Surg. 2012 17(2):58-62. [Google Scholar]
[6]. P Vassallo, G Edel, N Roos, A Naguib, PE Peters, In-vitro high-resolution ultrasonography of benign and malignant lymph nodes. A sonographicpathologic correlation.Invest Radiol. 1993 28:698-705. [Google Scholar]
[7]. PM Som, Lymph nodes of the neck.Radiology. 1987 165:593-600. [Google Scholar]
[8]. M Ying, A Ahuja, F Brook, Sonographic appearances of cervical lymph nodes: Variations by age and sex.J Clin Ultrasound. 2002 30:1-11. [Google Scholar]
[9]. AT Ahuja, L Chow, W Chick, W King, C Metreweli, Metastatic cervical nodes in papillary carcinoma of the thyroid: ultrasound and histological correlation.Clin Radiol. 1995 50:229-31. [Google Scholar]
[10]. EW To, WM Tsang, J Cheng, E Lai, P Pang, AT Ahuja, M Ying, Is neck ultrasound necessary for early stage oral tongue carcinoma with clinically N0 neck?Dentomaxillofac Radiol. 2003 32(3):156-59. [Google Scholar]
[11]. Aggarwal Ankur, Daniel M Jonathan, Srinivasan SV, Sargouname Charles P, Gray scale ultrasonography in the assessment of regional lymphnodes in oral cancer and its correlation with TNM staging and FNAC.Journal of Indian Academy of Oral Medicine and Radiology. 2011 23(2):104-07. [Google Scholar]
[12]. A Ahuja, M Ying, R Evans, W King, C Metreweli, The application of ultrasound criteria for malignancy in differentiating tuberculous cervical adenitis from metastatic nasopharyngeal carcinoma.Clin Radiol. 1995 50:391-95. [Google Scholar]
[13]. M Ying, AT Ahuja, R Evans, W King, C Metreweli, Cervical lymphadenopathy: sonographic differentiation between tuberculous nodes and nodal metastases from non-head and neck carcinomas.J Clin Ultrasound. 1998 26:383-89. [Google Scholar]
[14]. S Vivek, KS Siva, Enlarged lymph nodes in head and neck cancer: Analysis with triplex ultrasonography.Annals of Maxillofacial Surgery. 2013 3(1):35-9. [Google Scholar]