Comparative Efficacy and Safety of Triple Therapy (Ramipril, Telmisartan, Hydrochlorothiazide) Vs Dual Anti Hypertensive Therapy (Ramipril or Telmisartan, Hydrochlorothiazide) in Stage 2 Hypertensive Patients
Bharat Bhushan1, Seema Gupta2, Vijay Khajuria3, Dinesh Kumar4, Mohan Lal5, Dharminder Kumar6, Sanjeev Bhat7, Aman Sharma8
1 Resident, Department of Pharmacology, Government Medical College, Jammu, India.
2 Assistant Professor, Department of Pharmacology, Government Medical College, Jammu, India.
3 Associate Professor, Department of Pharmacology, Government Medical College, Jammu, India.
4 Associate Professor, Department of Preventive and Social Medicine, Government Medical College, Jammu, India.
5 Professor, Department of Cardiology, Government Medical College, Jammu, India.
6 Assistant Professor, Department of Cardiology, Government Medical College, Jammu, India.
7 Lecturer, Department of Cardiology, Government Medical College, Jammu, India.
8 Resident, Department of Pharmacology, Government Medical College, Jammu, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Bharat Bhushan, H.No. F-65, Upper Krishna Nagar, Near Resham Ghar Colony, Jammu 180016, India. Phone : 9086065555, 9858911910, E-mail : bharat4rl@yahoo.com
Aim: To evaluate the comparative efficacy and safety of ramipril 5mg plus hydrochlorothiazide 12.5mg (R + HCTZ), telmisartan 40mg plus hydrochlorothiazide12.5mg (T + HCTZ) and ramipril 2.5mg plus telmisartan 20mg plus hydrochlorothiazide12.5mg (R + T + HCTZ) in patients with stage 2 hypertension.
Materials and Methods: A prospective, open label, randomized comparative study was conducted to study the comparative efficacy and safety of R+HCTZ (group 1), T+HCTZ (group 2)and R+T+TCTZ (group3) in 88 patients with stage 2 hypertension without co-morbid conditions. Echocardiography was done to assess left ventricular function. Patients were followed up to 24 weeks and any ADR occurring in this period was recorded.
Results: All the three treatment groups showed significant fall in both systolic and diastolic blood pressure compared to the baseline scores (p<0.0001). Intergroup comparison did not reveal any significant difference. Total number of adverse drug events reported were 15. Group III had higher percentage ADRs. Dry cough (8) was most common ADR. The echocardiography parameters did not change from baseline values with all three treatment regimens.
Conclusion: All three medications were of equal efficacy in patients with stage 2 hypertension without co morbid conditions, failing to prove superiority over each other.
Hydrochlorothiazide, Ramipril, Stage 2 hypertension, Telmisartan, Triple therapy
Introduction
Hypertension is a growing global health problem and is predicted to affect 1.56 billion people by 2025. The poorly controlled hypertension can damage target organs, eventually resulting in heart failure, end-stage kidney disease, retinopathy and vascular dementia.
The 7th Report of US Joint National Commission on Prevention, detection, evaluation and treatment of high blood pressure has classified hypertension as Prehypertension as SBP 120-139 mmHg or DBP 80-89 mmHg, whereas Hypertension, Stage 1 is defined as SBP 140-159 mmHg or DBP 90-99 mmHg while Stage 2 is defined as SBP ≥160 mmHg or DBP ≥100 mmHg. Treatment goals recommended by JNC7 should achieve blood pressure levels <140/90 mmHg, or <130/80 mmHg for patients with co morbid conditions like diabetes or chronic renal disease [1].
Various classes of antihypertensive drugs including diuretics, beta blockers, angiotensin converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), calcium channel blockers (CCBs) and renin inhibitors are used for the treatment as monotherapy or in combination. Monotherapy is not always sufficient to achieve blood pressure control and combination therapy with at least two drugs, including a thiazide diuretic is recommended in patients with stage 2 hypertension [2]. In patients in whom dual therapy is inadequate, triple-drug therapy can be an alternative.
Clinical trials have reported that 23-52% patients require three or more antihypertensive agents for blood pressure control and target level maintenance. Thus, a triple drug combination therapy would be a desirable option in high risk hypertension [3].
Most of the studies with triple therapy with dual blockade of RAAS have been reported in patients of hypertension with co morbid conditions like diabetes mellitus and heart failure. There is a scarcity of research work elucidating the effect of dual RAAS blockers with thiazides in patients of hypertension stage 2 without co morbid conditions and whatever the data is available in literature is mostly from the west [4–7]. Therefore, present study was undertaken to evaluate the triple therapy regimen (dual RAAS block plus thiazide) for efficacy and safety in stage 2 hypertension and compare it with dual therapy comprising of ACEI (Ramipril) or ARB (Telmisartan) with thiazide.
Materials and Methods
The current prospective, randomized, open label, comparative, parallel study was conducted in the Department of Pharmacology in collaboration with Department of Cardiology, Government Medical College, Jammu, India starting w.e.f 1st November, 2011 for a period of one year. Study was approved by the Institutional Ethics Committee under number IEC/Pharma/17A/2011/2060 dated 20.10.2011. Written informed consent was obtained from all patients.
All the patients in the age group 18-60 years of both sexes attending Cardiology OPD diagnosed as new cases of stage 2 hypertension (Systolic Blood Pressure ≥ 160 mmHg and/or Diastolic Blood Pressure ≥ 100 mmHg) were included for the study. Patients with Stage 1 hypertension, Malignant or secondary hypertension, associated diabetes mellitus, ischemic heart disease, renal failure, bilateral renal artery stenosis or single kidney, hyperkalemia, heart failure,pregnancy and lactating mothers were excluded.
Study Design
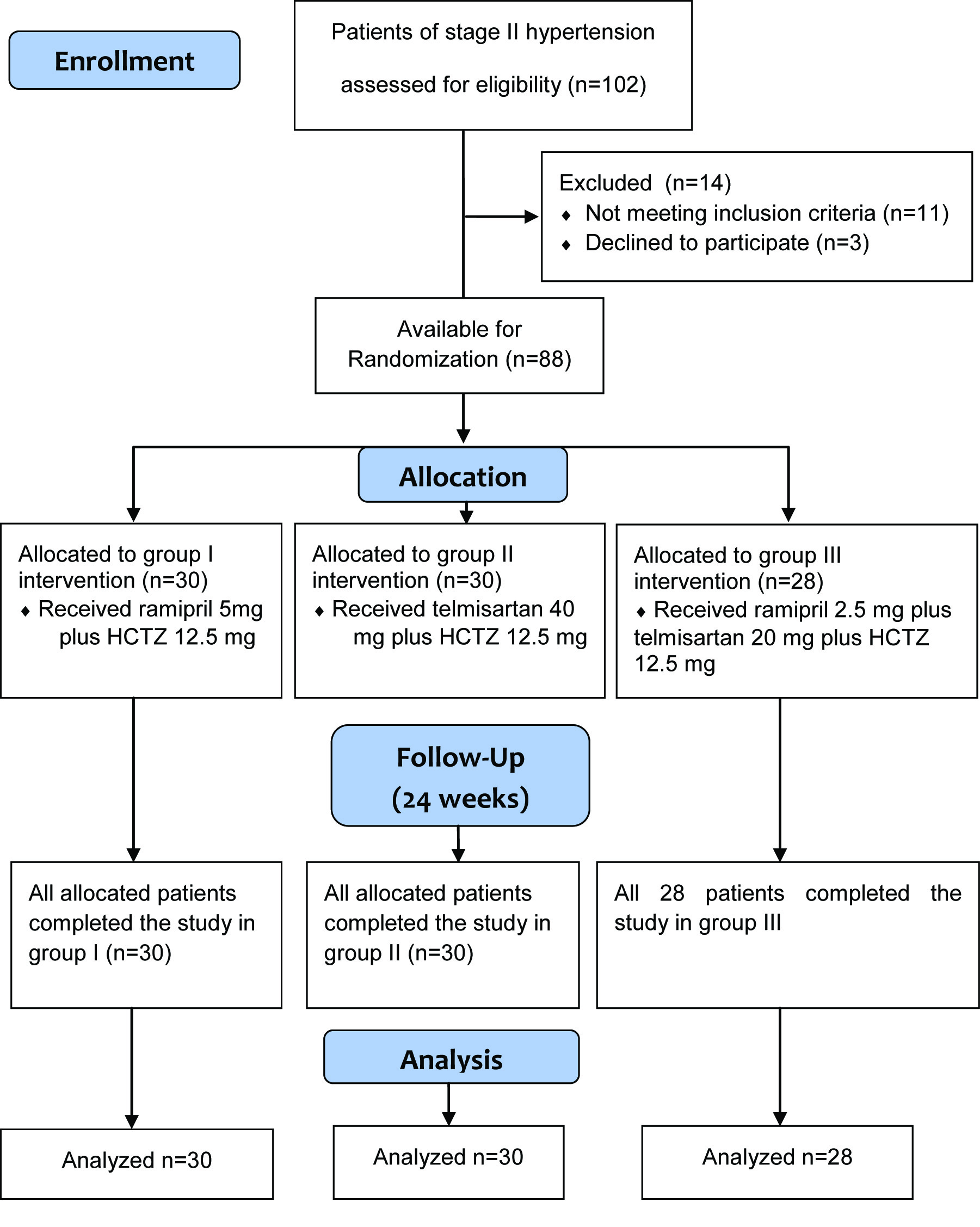
One hundred and two patients with hypertension were assessed for eligibility and finally 88 patient were enrolled as 14 did not meet inclusion criteria or declined to participate [Table/Fig-1]. A detailed medical history and complete examination was carried out at the time of enrollment for baseline values. Baseline characteristics are depicted in [Table/Fig-2]. Patients enrolled were randomized into three groups.
Baseline Characteristics of Patients (Mean±SEM)
| Characteristic | Group I (R+HCTZ) (n=30) | Group II (T+HCTZ) (n=30) | Group III (R+T+HCTZ) (n=28) |
|---|
| Sex | M (n = 21) | F (n = 9 | M (n = 18) | F (n = 12) | M (n = 16) | F (n = 12) |
| Age (yrs) | 49±5.1 | 53±3.7 | 52±4.7 | 49±6.1 | 48±5.9 | 51±2.8 |
| SBP (mmHg) | 159.6±1.9 | 158.2±2.0 | 158.9±2.1 |
| DBP (mmHg) | 100.7±1.0 | 99.5±0.9 | 98.9±1.1 |
| Pulse (beats/min) | 73±5.4 | 76±4.3 | 71±5.3 |
| Serum urea (mg/dl) | 33±4.9 | 31±6.2 | 34±5.7 |
| Serum creatinine (mg/dl) | 1.1±0.3 | 1.1±0.4 | 1.1±0.3 |
| Blood sugar (Fasting) (mg/dl) | 94.5±5.3 | 90.4±4.6 | 91.7±6.5 |
| Blood sugar (Post prandial) (mg/dl) | 110.2±9.7 | 113.1±6.1 | 108.4±8.8 |
SEM= standard error of mean, n= number of patients, M= male, F= female, yrs= years, SBP= systolic blood pressure, DBP= diastolic blood pressure
Group I (R+HCTZ) comprised of 30 patients were given tab. Ramipril 5 mg plus Hydrochlorothiazide 12.5 mg P.O. once a day. While Group II (T+ HCTZ) too had 30 patients and were treated with tab. Telmisartan 40 mg plus Hydrochlorothiazide 12.5 mg P.O. once a day. Whereas, Group III (R+T+ HCTZ) consisted of 28 patients and received Tab. Ramipril 2.5 mg plus Telmisartan 20 mg plus Hydrochlorothiazide 12.5 mg P.O. once a day. Each patient was followed up for a period of 24 weeks. All the patients attended six follow up visits at 4, 8, 12, 16, 20 and 24 weeks.
During each visit the systolic and diastolic blood pressure measure -ment were done by the auscultatory method. Two measurements were made with the standard mercury sphygmomanometer and the average was recorded [1].
Echocardiography was done at 0, 3 and 6 months to assess left ventricular function. Assessment of the degree of left ventricle hypertrophy (LVH) was done by measuring left ventricular posterior wall and interventricular septal thickness. Using 2-D echocardiogram as a guideline M-mode recording was obtained .Safety assessments consisted of regular monitoring and recording of all adverse drug events Adverse drug reactions (ADR) were reported using the form issued by Central Drugs Central Control Organization.
Statistical Analysis
Data was analysed using computer SPSS version 17.0 for Windows. Mean ± SEM were calculated. Statistical significance among three groups was assessed by one way Analysis of Variance (ANOVA). Posthoc, Bonferroni test was applied to evaluate inter-group significance. Paired t-test was used to evaluate statistical significance within a group. A p-value of equal to or less than 0.05 was considered as statistically significant except for paired t-test where Bonferroni correction was used to account for multiple comparisons. Chi-Square test was used to evaluate significance in the occurrence of adverse events.
Results
All 3 groups showed significant fall in systolic and diastolic blood pressure (p < 0.0001) from the pre drug baseline values, but the intergroup comparisons revealed no statistical significant difference amongst them [Table/Fig-3,4].
Comparative effect of the three treatment regimens on mean systolic blood pressure(mm of Hg)
| Groups | Weeks |
|---|
| 0 | 4 | 8 | 12 | 16 | 20 | 24 |
|---|
| I (n=30) | 159.6 | 142.2* | 139.8* | 138.4* | 136.3* | 133* | 131* |
| II(n=30) | 158.2 | 142.4* | 139.9* | 136.8* | 134.5* | 133.6* | 131.1* |
| III(n=28) | 158.9 | 137.5* | 136.6* | 131.9* | 129.6* | 127.9* | 126.1* |
| NS | NS | NS | NS | NS | NS | NS |
Group I (R+HCTZ) group II(T+HCTZ) group III (R+T+HCTZ). n is number of patients *p< 0.0001 as compared to the baseline values (0wk) within each group, Intergroup comparison between three groups– non significant (NS)
Comparative effect of the three treatment regimens on mean diastolic blood pressure (mm of Hg)
| Groups | Weeks |
|---|
| 0 | 4 | 8 | 12 | 16 | 20 | 24 |
|---|
| I (n=30) | 100.7 | 94.2* | 92* | 90.2* | 88.4* | 87* | 86.4* |
| II (n=30) | 99.5 | 93.2* | 91.8* | 86.2* | 85* | 83.8* | 83.1* |
| III(n=28) | 98.9 | 92.9* | 92.4* | 90.7* | 88.9* | 86.6* | 85.3* |
| NS | NS | NS | NS | NS | NS | NS |
Group I (R+HCTZ) group II(T+HCTZ) group III (R+T+HCTZ). n is number of patients *p< 0.0001 as compared to the baseline values (0wk) within each group, Intergroup comparison between three groups– non significant (NS)
Echocardiography failed to record any significant alterations (p>0.05)in values from base line values on left ventricle posterior wall thickness and interventricular septal thickness in all three groups up to 24 weeks [Table/Fig-5,6].
Comparative effect of the three treatment regimens on left ventricular posterior wall thickness in mm (Mean±SEM)
| Months | Group I n=30 | Group II n=30 | Group III n=28 |
|---|
| 0 | 9.63±0.45 | 9.96±0.48 | 9.25±0.45 |
| 3 | 9.60±0.41 NS | 9.50±0.36 NS | 9.03±0.38NS |
| 6 | 9.56±0.41 NS | 9.26±0.36 NS | 9.03±0.35 NS |
Group I (R+HCTZ) group II(T+HCTZ) group III (R+T+HCTZ) n is number of patients Compared to the baseline values (0wk) within each group and Intergroup comparison between three groups– non significant (NS)
Comparative effect of the three treatment regimens on interventricular septal thickness in mm (Mean±SEM)
| Months | Group I n=30 | Group II n=30 | Group III n=28 |
|---|
| 0 | 9.70±0.45 | 9.80±0.41 | 9.39±0.45 |
| 3 | 9.30±0.39 NS | 9.40±0.33 NS | 9.07±0.37NS |
| 6 | 9.13±0.33 NS | 9.23±0.33 NS | 9.07±0.35 NS |
Group I (R+HCTZ) group II(T+HCTZ) group III (R+T+HCTZ) n is number of patients Compared to the baseline values (0wk) within each group and Intergroup comparison between three groups– non significant (NS)
Total number of adverse drug events reported by the patients during the entire study were 15 [Table/Fig-7]. ADRs were reported by spontaneous as well as by intensive reporting. Intergroup comparisons did not reveal statistical significant difference amongst them using chi square test (p= 0.138).
Number of ADR events in three groups
| Group I n=30 | Group II n=30 | Group III n=28 |
|---|
| Headache | 0 | 1 | 2 |
| Dry cough | 3 | 1 | 4 |
| Hyperglycemia | 1 | 0 | 0 |
| Hypotension | 0 | 0 | 2 |
| Hyperkalemia | 0 | 0 | 1 |
| Total | 4 (26.67%) | 2 (13.33%) | 9 (60%) |
Group I (R+HCTZ) group II(T+HCTZ) group III (R+T+HCTZ) n is number of patients. Intergroup comparison between three groups non significant (p=0.138) using chi square test
Discussion
Recently combining diuretic and drugs blocking RAAS in the treatment of hypertension has gained interest but a lot of conflicting results have been reported [7,8]. The present study the efficacy and tolerability of dual and triple drug combination (including two RAAS blocking agents) were compared. Results in group I revealed significant fall in both SBP and DBP (p < 0.0001). Various studies have demonstrated fall in blood pressure in response to combination of a diuretic and an angiotensin-converting enzyme inhibitor (ACEI) or angiotensin receptor blocker [9–12].
Number of authors have shown decline in blood pressure both SBP as well as DBP with ramipril with hydrochlorothiazide combination than their respective components [13–15]. The rationale for using the ACE inhibitors or ARBs in combination with thiazide diuretics has centered around antihypertensive synergy and counterbalancing adverse metabolic effects [16].
In the present study, group II revealed significant fall in SBP and DBP (p<0.0001) and these results are in accordance with earlier studies [17,18]. The combination has been documented to be more effective in patients who inadequately respond to telmisartan alone [18–20]. Amongst ARBs telmisartan is known to effectively prevent the early morning rise of blood pressure and reduce cardiovascular and renal complications [21].
In the present study triple combination decreased both systolic and diastolic blood pressure (p < 0.0001). Only a few reports regarding the triple therapy regimen in stage 2 hypertension are available. Triple therapies with amlodipine / valsartan / hydrochlorothiazide [22] and olmesartan 40 mg plus amlodipine 10 mg plus hydrochlorothiazide 25 mg reported to reduced blood pressure significantly more than their dual combinations [23]. Comparison of triple therapy did not reveal statistical significant fall in blood pressure than dual drug combinations . The possible explanation for the similar efficacy may be because of low dose of ramipril and telmisartan used in group III .Our results are in accordance with ONTARGET trial [7].
Both ACE inhibitors and ARBs are generally well tolerated. The major side effects are cough, hyperkalemia and less often angioedema [24]. In the present study, dry cough was the most common ADR event. Maximum number of dry cough occurred in groups consisting of ramipril. This implies that probably ramipril was the common offending drug as it leads to accumulation of bradykinin, substance P, and/or prostaglandins in the lungs. Cough was less common with ARBs as one event reported in group II.
The ONTARGET trial [7] found a significant increase in adverse effects with combined therapy compared to ACE inhibitors alone. The present study revealed similar findings where maximum ADRs were in group III. TRANSCEND trial [25] also showed that ramipril alone or with telmisartan, was associated with a higher proportion of discontinuations due to adverse events.
Since the fall of blood pressure (SBP and DBP) was similar in all 3 groups meaning thereby that all the drug combinations were of equal efficacy but the safety profile appeared more favourable in group I and II over group III. This makes the group I and II with dual combination therapy preferred option.
Patients with hypertension are at increased risk of developing a variety of cardiac structural and functional changes [26]. However, left ventricular parameters did not reveal any significant alteration from the mean baseline suggesting no further deterioration during study.
Conclusions
From the foregoing discussion we conclude that triple antihyper- tensive therapy failed to elicit the advantage as far efficacy was concerned over dual drug therapy in stage 2 hypertension patients without co-morbid conditions. All regimens were well tolerated. However, while generalization of results on safety, caution must be exercised as sample size was small and a few adverse events were recorded. Therefore further studies are suggested with large sample size to address the safety issue.
SEM= standard error of mean, n= number of patients, M= male, F= female, yrs= years, SBP= systolic blood pressure, DBP= diastolic blood pressure
Group I (R+HCTZ) group II(T+HCTZ) group III (R+T+HCTZ). n is number of patients *p< 0.0001 as compared to the baseline values (0wk) within each group, Intergroup comparison between three groups– non significant (NS)
Group I (R+HCTZ) group II(T+HCTZ) group III (R+T+HCTZ). n is number of patients *p< 0.0001 as compared to the baseline values (0wk) within each group, Intergroup comparison between three groups– non significant (NS)
Group I (R+HCTZ) group II(T+HCTZ) group III (R+T+HCTZ) n is number of patients Compared to the baseline values (0wk) within each group and Intergroup comparison between three groups– non significant (NS)
Group I (R+HCTZ) group II(T+HCTZ) group III (R+T+HCTZ) n is number of patients Compared to the baseline values (0wk) within each group and Intergroup comparison between three groups– non significant (NS)
Group I (R+HCTZ) group II(T+HCTZ) group III (R+T+HCTZ) n is number of patients. Intergroup comparison between three groups non significant (p=0.138) using chi square test
[1]. The Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Cited 2011 September 23. Available from www.nhlbi.nih.gov/guidelines/hypertension [Google Scholar]
[2]. Wright RF, Duprez D, Purkayastha D, Samuel R, Ferdinand KC, Combination Angiotensin-Receptor Blocker (ARB)/Calcium Channel Blocker With HCTZ versus the Maximal Recommended Dose of an ARB With HCTZ in Patients With Stage 2 Hypertension: The Exforge As Compared to Losartan Treatment in Stage 2 Systolic Hypertension (EXALT) StudyJ Clin Hypertens 2011 13:588-97. [Google Scholar]
[3]. Destro M, Crikelair N, Yen J, Glazer R, Triple combination therapy with amlodipine, valsartan, and hydrochlorothiazide versus dual combination therapy with amlodipine and hydrochlorothiazide for stage 2 hypertensive patientsVasc Health Risk Manag 2010 6:821-27. [Google Scholar]
[4]. Mogensen CE, Neldam S, Tikkanen I, Oren S, Viskoper R, Watts RW, Randomised controlled trial of dual blockade of renin–angiotensin system in patients with hypertension, microalbuminuria, and non-insulin dependent diabetes: the candesartan and lisinopril microalbuminuria (CALM) studyBr Med J 2000 321:1440-44. [Google Scholar]
[5]. Nakao N, Yoshimura A, Morita H, Takada M, Ideura T, Combination treatment of angiotensin-II receptor blocker and angiotensin-converting-enzyme inhibitor in non-diabetic renal disease (COOPERATE): a randomised controlled trialLancet 2003 361:117-24. [Google Scholar]
[6]. Velazquez EJ, Pfeffer MA, McMurray JV, Maggioni AP, Rouleau JL, Van de Werf F, VALsartan In Acute myocardial iNfarcTion (valiant) trial: baseline characteristics in contextEur J Heart Fail 2003 5(4):537-44. [Google Scholar]
[7]. Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, ONTARGET investigators. Telmisartan, ramipril, or both in patients at high risk for vascular eventsN Engl J Med 2008 358:1547-59. [Google Scholar]
[8]. Geiger H, Barranco E, Gorostidi M, Taylor A, Zhang X, Xiang Z, Zhang J, Combination Therapy With Various Combinations of Aliskiren, Valsartan, and Hydrochlorothiazide in Hypertensive Patients Not Adequately Responsive to Hydrochlorothiazide AloneThe Journal of Clinical Hypertension 2009 11(6):324-32. [Google Scholar]
[9]. Weinberger MH, Comparison of captopril and hydrochlorothiazide alone and in combination in mild to moderate essential hypertensionBr J Clin Pharmacol 1982 14(2):127S-31S. [Google Scholar]
[10]. Chrysant SG, The lisinopril-hydrochlorothiazide group. Antihypertensive effectiveness of low dose hydrochlorothiazide combination. A large multicentre studyArch Intern Med 1994 154:737-43. [Google Scholar]
[11]. Kochar M, Guthrie R, Triscari J, Kassler-Taub K, Reeves RA, Matrix study of irbesartan with hydrochlorothiazide in mild-to-moderate hypertensionAm J Hypertens 1999 12:797-805. [Google Scholar]
[12]. Ruilope LM, Clinical efficacy and safety of olmesartan/ hydrochlorothiazide combination therapy in patients with essential hypertensionVasc Health Risk Manag 2008 4(6):1237-48. [Google Scholar]
[13]. Heidbreder D, Froer KL, Breitstadt A, Cairns V, Langley A, Bender N, Combination of ramipril and hydrochlorothiazide in the treatment of mild to moderate hypertension: Part 1--A double-blind, comparative, multicenter study in nonresponders to ramipril monotherapyClin Cardiol 1992 15(12):904-10. [Google Scholar]
[14]. Scholze J, Breitstadt A, Cairns V, Bauer B, Bender N, Priestley C, Short report: ramipril and hydrochlorothiazide combination therapy in hypertension: a clinical trial of factorial design. The East Germany Collaborative Trial GroupJ Hypertens 1993 11(2):217-21. [Google Scholar]
[15]. Genthon R, Study of the efficacy and safety of the combination ramipril 2.5 mg plus hydrochlorothiazide 12.5 mg in patients with mild-to-moderate hypertension. ATHES Study GroupInt J Clin Pharmacol Res 1994 14(1):1-9. [Google Scholar]
[16]. Waeber B, Combination therapy with ACE inhibitors/angiotensin II receptor antagonists and diuretics in hypertensionExpert Review of Cardiovascular Therapy 2003 1(1):43-50. [Google Scholar]
[17]. McGill JB, Paul A, ReillyTelmisartan plus hydrochlorothiazide versus telmisartan or hydrochlorothiazide monotherapy in patients with mild to moderate hypertension: a multicenter, randomized, double-blind, placebo-controlled, parallel-group trialClinical Therapeutics 2001 23(6):833-50. [Google Scholar]
[18]. Chen SX, Zhang J, Chen SL, Chen JZ, Yan XW, Ke YN, A randomized, double-blind, double-dummy study comparing a fixed dose combination of telmisartan 80 mg plus hydrochlorothiazide 12.5 mg to telmisartan 80 mg in Chinese hypertensive patients who failed to respond adequately to telmisartan 80 mgZhonghua Xin Xue Guan Bing ZaZhi 2008 36(4):300-04. [Google Scholar]
[19]. Neutel JM, Littlejohn TW, Chrysant SG, Singh A, Telmisartan / Hydrochlorothiazide in Comparison with Losartan / Hydrochlorothiazide in Managing Patients with Mild-to-Moderate HypertensionJ Hypertension Research 2005 28:555-63. [Google Scholar]
[20]. Sharma AM, Davidson J, Koval S, Lacourcière Y, Telmisartan/hydrochlorothiazide versus valsartan/hydrochlorothiazide in obese hypertensive patients with type 2 diabetes: the SMOOTH studyCardiovasc Diabetol 2007 6:28 [Google Scholar]
[21]. Chrysant SG, Chrysant GS, Desai A, Current status of angiotensin receptor blockers for the treatment of cardiovascular diseases: focus on telmisartanJournal of Human Hypertension 2005 19:173-83. [Google Scholar]
[22]. Calhoun DA, Lacourciere Y, Chiang YT, Glazer RD, Triple antihypertensive therapy with amlodipine, valsartan, and hydrochlorothiazide. A randomized clinical trialHypertension 2009 54:32-39. [Google Scholar]
[23]. Oparil S, Melino M, Lee J, Fernandez V, Heyrman R, Triple therapy with olmesartan medoxomil, amlodepine besylate and hydrochlorothiazide in adult patients with hypertension. The trinity multicentral randomized double blind 12 weeks parallel studyClin Ther 2010 32(7):1252-69. [Google Scholar]
[24]. Izzo JL, Weir MR, Angiotensin-converting enzyme inhibitorsJ Clin Hypertens 2011 13:667 [Google Scholar]
[25]. Teo K, Yusuf S, Sleight P, Anderson C, Mookadam F, Ramos B, The TRANSCEND Investigators. Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high risk patients intolerant to angiotensin converting enzyme inhibitors: a randomized controlled trialThe Lancet 2008 372:1174-83. [Google Scholar]
[26]. Mitchell GF, Vasan RS, Keyes MJ, Parise H, Wang TJ, Larson MG, Pulse pressure and risk of new-onset atrial fibrillationJAMA 2007 297(7):709-15. [Google Scholar]