Introduction
In daily practice dentists frequently come across white lesions in the oral cavity, to the extent of about 24.8% [1]. Among oral lesions, oral cancer is a major health problem in world with its high mortality rate and is seen mainly in developing countries like Indian subcontinent [2]. The two step concept for development of cancer has been in practice since a long time, suggesting that cancer initially presents as a precancer precursor which subsequently transforms into a frank cancer. Hence, generally but not always oral cancer is preceded by premalignant lesions like leukoplakia, erythroplakia or premalignant conditions like lichen planus, oral submucous fibrosis. These terminologies now have been replaced with term potentially malignant disorders [3].
Leukoplakia (leukos meaning white; plakia meaning patch) is a clinical term which is based on exclusion criterion after excluding other white lesions like lichen planus, leukoedema, white sponge nevus, etc [4]. It is the most common potentially malignant disorder affecting the oral mucosa [5]. Schwimmer in 1877 coined the term leukoplakia in 1978 and since then the definition of leukoplakia has been modified. WHO (1978) defined it as “ A white patch on the oral mucosa that can neither be scrapped off nor classified as any other diagnosable disease”. Later on in 1984, the definition was modified adding “oral leukoplakia is not associated with any physical or chemical causative agent except the use of tobacco”. Later on in 1986, oral leukoplakia was defined as “a predominantly white lesion of the oral mucosa that cannot be characterized as any other definable disease”. Then in 1997, “any other definable lesion” was used in the definition instead of “any other definable disease”. Recently, WHO (2005) changed the definition of leukoplakia as “a white plaque of questionable risk having excluded (other) known diseases or disorders that carry no increased risk for cancer” [3–5].
Epidemiology: Various studies have shown the prevalence of leukoplakia to be between 0.2 to 3.6%, with regional variations like in India (0.2-4.9%), Sweden (3.6%), Germany (1.6%) and Holland (1.4%) [6–8].
Aetiology and Pathogenesis
Tobacco in various forms was found to be the chief aetiologic factor for leukoplakia. Tobacco contains many carcinogens which are collectively called as tar, and were found to be toxic and carcinogenic. Smokers have an increased risk of developing leukoplakia than nonsmokers, as studies have shown that more than 80% of leukoplakia patients were smokers. People who smoke heavily were found to have multiple and larger sized lesions than who smoke less. They found that the lesion either subsided totally or became smaller after cessation of smoking habit [9–12].
Apart from tobacco, alcohol consumption was found as a common habit in patients with leukoplakia. Generally, alcohol and tobacco are consumed together by individuals. Even though alcohol alone was not found to be associated with development of leukoplakia, it was found to have some synergistic effect with tobacco in the development of both leukoplakia and oral cancer [13]. Mechanical trauma in the form of chronic cheek biting, ill-fitting dentures, etc have also found to be contributory factors for leukoplakia. Experimental studies on animals have shown that application of carcinogens to the traumatized mucosa resulted in transformation of epithelial cells to dysplastic cells and leukoplakia like lesions in such areas may also be a protective response to trauma [14,15].
Leukoplakia and Sanguinaria: Herbal extract sanguinaria which is used in mouth washes and tooth pastes was found to develop leukoplakia (Sanguinaria-associated keratosis). Even after stopping usage of this product, the lesion did not subside. The commonest site was maxillary vestibule and alveolar mucosa [4].
Leukoplakia and Candida: Studies have been carried out to find out the association between leukoplakia and candida and in few leukoplakias, nitrosamine producing Candida species were found. They found that even after elimination of surface mycosis after administration of antifungals, the leukoplakia persisted. They also noted that the malignant transformation of candida infected leukoplakias was high, suggesting candida association as a significant risk factor for oncogenesis [16].
Leukoplakia and Papilloma virus: Extensive molecular biology and virology studies have been carried out to find out the role of Human Papilloma Virus (HPV) in the aetiology as well as oncogenesis of oral leukoplakia. HPV type 16 was demonstrated in oral leukoplakias and carcinomas. In a more aggressive variant of leukoplakia, Proliferative Verrucous leukoplakia (PVL), HPV 16 and 18 were isolated [16].
Leukoplakia and Epstein Barr virus (EBV): Even though EBV was found to be associated with aetiology of oral squamous cell carcinomas, their role in oral leukoplakias was not found in any of the studies. May be carrying out studies on a larger sample may help us if there is any role of EBV in oral leukoplakias [16].
Clinical Features Age: Most of the patients with leukoplakia were over 40 years of age, mainly seen in fifth to seventh decades with average age to be 60 y. Its prevalence was higher with age in males [4].
Sex: Leukoplakia is seen mainly in males with a ratio of 2:1 [16,17].
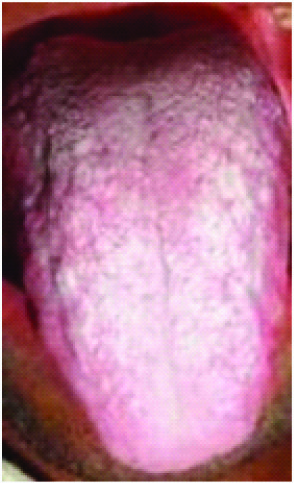
Site: Leukoplakia is commonly seen on lips, buccal mucosa, tongue and gingiva. The site varies with the form of tobacco habit, like in beedi smokers the site was anterior buccal mucosa where as in patients who chew tobacco, seen on the posterior buccal mucosa [Table/Fig-1,2] [18].
Leukoplakia on dorsal surface of tongue
Leukoplakia on buccal mucosa

Colour: Generally it is seen as gray, white or yellowish white in color [17].
Clinical Appearance: Leukoplakia presents a diverse clinical appearance and with time its appearance often changes. Usually it takes about 2.4 y to diagnose the lesion. Initially the lesion appears as a thin, slightly elevated gray or grayish white translucent plaque. The lesion is characteristically soft and flat and is sometimes wrinkled or fissured. The borders of the lesion are usually sharply demarcated but rarely some lesions blend gradually into adjacent normal mucosa. Some authors have designated the term preleukoplakia to this early stage, few others have preferred to use this stage as thin leukoplakia. Later, the lesions become thicker, extend laterally and become more whitish in colour. The fissures may become deepen and leathery on palpation. This stage is referred as thick or homogenous leukoplakia. Some severe lesions develop surface irregularaties and are designated as granular or nodular leukoplakia. Verrucous leukoplakias show sharp or blunt projections [4,16,17].
Based on the clinical appearance sharp described three stages or phases of leukoplakia:
Phase I: A white, slightly translucent non palpable lesion
Phase II: Later on the lesion develop as an opaque white, slightly elevated plaque with irregular outline. The lesion may be localized or diffuse and may have a granular texture.
Phase III: Then the lesion may progress to thickened white lesions that show fissuring, induration and ulcer formation.
Few authors have added phase IV, which include mixed red and white lesions and designated terms erythroleukoplakia, speckled leukoplakia or non homogeneous leukoplakia. These lesions have been shown to have a higher malignant transformation rates [17,18].
Clinical Variants
Pindborg [4] classified leukoplakia into two main types
Homogeneous leukoplakia
Non homogeneous leukoplakia
Bailoor and Nagesh [19] divided leukoplakia in to
- Speckled leukoplakia and non speckeled leukoplakia
- Homogenous, Ulcerative, Speckled
- Reversible / irreversible
WHO [4] (1980) subdivided leukoplakia into various forms [Table/Fig-3].
Leukoplakia classification (WHO 1980)
| HOMOGENEOUS | NONHOMOGENEOUS |
|---|
| Smooth | Nodulospeckled |
| Furrowed (Fissured) | |
| Ulcerated | |
Homogeneous leukoplakia/Leukoplakia simplex: Lesions are uniformly flat, thin and predominantly white in colour. The surface of the lesion may be smooth, wrinkled or corrugated and with a consistent texture throughout. These lesions are asymptomatic and show a very low risk of malignant transformation [4,16,17].
Non Homogeneous Leukoplakia/Erythroleukoplakia: Mixed white and red lesions associated with an erythematous component. Patients complain of pain, itching and discomfort. These show a high risk for malignant transformation [4,16,17].
Proliferative Verrucous Leukoplakia (PVL): PVL is an aggressive variant of leukoplakia, first described by Hansen et al., in 1985. It shows a female preponderance in contrast to other subtypes of leukoplakia with female to male ratio of about 4:1. In patients with PVL, smoking and drinking were not found to be significant. The commonest site in females was buccal mucosa whereas in males tongue was frequently involved. The significance of PVL is that the lesions show high risk for malignant transformation, treatment resistant and show high recurrence rates. Hence such lesions require early and aggressive treatment. HPV 16 was found to be associated with this lesion. Four stages have been described in its development, initially as a simple hyperkeratosis without epithelial dysplasia, followed by verrucous hyperplasia, verrucous carcinoma, and finally conventional carcinoma [20–22].
Ghazali et al., [23] suggested the following criteria for the diagnosis of PVL:
The lesion should start as homogeneous leukoplakia with histopathological findings of dysplasia
Later in it should show verrucous areas
From single lesion it should progress to multiple lesions at the same or different site
It should progress later into different histological stages.
It should show recurrence after treatment
Oral Hairy Leukoplakia (OHL): OHL is a white lesion related to Epstein-Barr virus (EBV). It is usually associated with AIDS. OHL is seen on lateral border of the tongue, rarely on the buccal mucosa, with slightly raised and corrugated hairy surface. Like leukoplakia these lesions are also white in colour, cannot be rubbed off and asymptomatic. But OHL must not be considered as a variant of leukoplakia as its aetiological factor is EBV virus [11].
Diagnosis: Leukoplakias are diagnosed based on history and clinical examination. It is mandatory to biopsy all the lesions which are clinically suspected to be leukoplakias. Biopsy is done to confirm the diagnosis so that proper treatment can be planned. In large lesions, incisional biopsy should be performed including some adjacent normal tissue, where as if the lesion is small, excisional biopsy should be performed. To select the appropriate biopsy site toluidine blue and vizilite are used. The primary significance of incisional biopsy in such lesions is to detect the presence or absence of dysplasia, grade of dysplasia if present, as dysplasia, carcinoma in situ or invasive carcinoma cannot be predicted clinically. Incisional biopsy is done if the lesion is large in size, if in inaccessible sites, at multiple sites, and mandatory if the lesion is non homogenous. It also helps in excluding other recognized white lesions. The site of the biopsy should be from symptomatic area and if the lesion is asymptomatic, it should be taken from red or indurated areas [4,17].
Differential Diagnosis: Lesions that must be included in differential diagnosis of leukoplakia should be lichen planus, leukoedema, white sponge nevus, syphilitic mucous patch, discoid lupus erythematosis, verruca vulgaris, chemical burn, and chronic cheek bite [4,17].
Histopathological Features- Basically, leukoplakia is a clinical term. The histopathological findings comprise epithelial hyperplasia and surface hyperkeratosis (hyperparakeratosis or hyperorthokeratosis). In some lesions epithelial dysplasia may be seen and may range from mild to severe, based on its presence leukoplakia is of two types dysplastic and non dysplastic [4,17]. The criterion used for dysplasia are listed in [Table/Fig-4] [24].
Criteria used for diagnosing dysplasia
| CYTOLOGY | ARCHITECTURE |
|---|
| Abnormal variation in nuclear size (anisonucleosis) | Irregular epithelial stratification |
| Abnormal variation in nuclear shape (nuclear pleomorphism) | Loss of polarity of basal cells |
| Abnormal variation in cell size (anisocytosis) | Drop-shaped rete ridges |
| Abnormal variation in cell shape (cellular pleomorphism) | Increased number of mitotic figures |
| Increased nuclear-cytoplasmic ratio | Abnormal superficial mitoses |
| Increased nuclear size | Premature keratinization in single cells (dyskeratosis) |
| Atypical mitotic figures | Keratin pearls within rete pegs |
| Increased number and size of nucleoli | Basal cell hyperplasia |
| Hyperchromasia | |
Verrucous leukoplakia shows papillary surface projections and broad reteridges, difficult to differentiate from verrucous carcinoma. PVL initially resemble leukoplakias but as the lesion progresses it resembles squamous cell carcinoma [4].
Modified Classification and Staging System
Based on size of the lesion and presence or absence of epithelial dysplasia, Van der Waal et al., proposed a four stage OLEP staging system [Table/Fig-5] [25].
Van der Waal et al., (2000) OLEP Classification and Staging System
| L-Size of the lesion | P-Pathology |
|---|
| L1 = size less than 2 cm | P0 = No Epithelial Dysplasia |
| L2= size 2 to 4 cm | P1 = Distinct Epithelial Dysplasia |
| L3= size greater than 4 cm | Px = Dysplasia not specified in the pathology report |
| Lx= size not specified | |
| OLEP Staging System |
| STAGE | FINDINGS |
| STAGE I | L1P0 |
| STAGE II | L2P0 |
| STAGE III | L3P0 or L1L2P1 |
| STAGE IV | L3P1 |
LCP STAGING
Based on size, clinical and pathological stages, LCP grading of leukoplakia was given [Table/Fig-6] [17].
Leukoplakia classification (WHO 1980)
| SIZE OF THE LESION | CLINICAL | PATHOLOGY |
|---|
| Lx- size not specified | C1- Homogenous | Px- Not specified |
| L1-less than 2 cm, single/ multiple | C2-Nonhomogenous | P0- No epithelial dysplasia |
| L2-2 to 4 cm, single/ multiple | | P1-Distinct epithelial dysplasia |
| L3-more than 4cm, single/ multiple | | |
| SIZE OF THE LESION | CLINICAL | PATHOLOGY |
|---|
| STAGE 1 | L1 P0 | L1 C1 |
| STAGE 2 | L2 P0 | L2 C1 |
| STAGE 3 | L3 P0 | L3 C1 |
| STAGE 4 | L3 P1 | L3 C2 |
Malignant Transformation Potential
Various studies have shown 0.6 to 20% rate of malignant transformation of leukoplakia. The factors that are thought to increase the transformation rate are [4,17,23].
Age: Transformation rates were found to be increasing with increasing age.
Size: Large size lesions (more than 20mm) showed high transformation rates.
Habits: Malignant transformation was found greater in smokers than non smokers.
Site: The risk of transformation varied with the site, high risk areas being floor of mouth and tongue, low risk areas being buccal mucosa and commissures.
Gender: Transformation rates were found to be higher in females (6%) than male (3.9%).
Clinical type: Non homogenous types and PVL showed higher rates than homogenous type.
Epithelial Dysplasia: Considered as the most important factor for malignant transformation. Dysplastic leukoplakias showed a higher risk of malignant transformation than non-dysplastic leukoplakias.
Candida: Leukoplakia with candida super infection showed higher malignant risk.
Biomarkers: Recent developments in the field of molecular biology have tremendously improved our knowledge about carcinogenesis, thus identifying the basic mechanisms leading to development of precancerous and cancerous lesions. Till now, the best predictor for malignant transformation of oral leukoplakia is presence of epithelial dysplasia, which has inter and intra examiner variability. But it was found that some dysplastic leukoplakias may remain unchanged or subside with time. Hence, few other parameters like DNA ploidy, p53 expression, HPV subtypes presence, and markers like podoplanin have been used to know the transformation rate and thus the prognosis of the lesion [16,18].
Loss of Heterozygosity: Loss of function of the allele of a gene whose homologous allele was earlier inactivated is referred to as loss of heterozygosity. Such phenomenon if occur in chromosomal regions with tumour suppressing genes was found to be related to malignant transformation. Zhang and Rosin reviewed the loss of heterozygosity in oral leukoplakia and categorized leukoplakia into high risk (loss from 3p and or 9p and loss from one or more of the 4q, 8p, 11q, 13q, and 17p chromosomes), intermediate risk (loss from 3p and or 9p) and low risk (no loss seen). High risk and intermediate risk lesions showed a 33 and 3.8 times chances of malignant transformation respectively than low risk lesions [16,18].
Aneuploidy: DNA ploidy or DNA content gives us the information about the extent of genetic stability and aberrations in the genomic sequence. In cancers, genetically unstable aneuploid cells replace the stable diploid cells. Flow cytometry techniques have been used to study to measure the ploidy status in oral leukoplakias and oral squamous cell carcinomas. They found that aneuploidy in dysplastic leukoplakia was a prognostic marker for malignant transformation of leukoplakia. They categorized dysplastic leukoplakias in to high risk (aneuploid lesions), intermediate risk (Tetraploid lesions) and low risk (diploid lesions). Further studies in larger samples must be carried out to determine the significance of this promising marker [16,18].
p53: p53 is a tumour suppressor gene which plays a vital role in DNA repair and cell cycle regulation. Mutation in this gene leads to cessation of the protective phenomenon and result in carcinogenesis. Studies have shown expression of p53 in more than 5% of the cells in oral leukoplakia [16,18].
Telomerase activity in Leukoplakia: Telomerase is an enzyme that lengthens the telomeres thus preventing cell apoptosis. Over expression of telomerase has been reported in leukoplakia correlating with dysplastic and cellular atypia changes [16,18].
Treatment: Counselling the patient to stop habits (tobacco or alcohol) is the primary step in the management of leukoplakia. The treatment may be conservative or surgical.
Conservative Treatment: Can be done by [26,27].
Enameloplasty to smoothen sharp teeth and replacement of faulty restorations to avoid trauma
Vitamin therapy (A, C and E) has a protective effect on the epithelium
Retinoids
Lycopene (a protein that interferes in cell cycle sequence by blocking the growth factor receptor signalling)
β carotenes (react with oxygen and form an unstable molecule, which is resistant to the action of oncogenic free radicals)
Nystatin therapy in case of candidal leukoplakia
Topical bleomycin, a cytotoxic antibiotic has been used in treatment of oral leukoplakia
Photodynamic therapy, which uses a photosensitising drug like Aminolaevulinic acid (ALA), oxygen and visible light. This causes destruction of exposed cells by a nonfree radical oxidative process.
Recurrences were seen after treating the patients conservatively. The treatment of the patients whether surgically or non surgically was based mainly on presence and extent of epithelial dysplasia.
Surgical Treatment: Various forms of surgical treatment include
Surgical excision is the treatment of choice and mostly performed procedure. Its main disadvantage is scar formation
Cryosurgery, with liquid nitrogen has been successfully used in treatment of leukoplakia, its principle of action being freezing of lesions
Laser therapy: studies have shown that Co2 laser therapy because of its excellent healing, lack of postoperative complications like bleeding and low recurrence rates is superior to other forms of treatment.
Follow up of the patients should be done frequently. Studies have shown that surgically treated patients have less chance of malignant transformation than those treated nonsurgically [28,29].
Conclusion
Oral leukoplakia is the most common potentially malignant disorder. The lesion can be diagnosed with the history and clinical examination. Biopsy of such lesions should be carried out and it should be differentiated with other white lesions. Early detection of leukoplakia is necessary as it shows high malignant transformation rates. New non invasive methods such as salivary markers in the detection of transformation should be carried out to control this lesion.
[1]. Axéll T, Occurrence of leukoplakia and some other oral white lesions among 20,333 adult Swedish peopleCommunity Dent Oral Epidemiol 1987 15(1):46-51. [Google Scholar]
[2]. Mehrotra R, Pandya S, Chaudhary AK, Kumar M, Singh M, Prevalence of Oral Pre-malignant and Malignant Lesions at a Tertiary Level Hospital in Allahabad, IndiaAsian Pac J Cancer Prev 2008 9:263-66. [Google Scholar]
[3]. Warnakulasuriya S, Johnson NW, van der Waal I, Nomenclature and classification of potentially malignant disorders of the oral mucosaJ Oral Pathol Med 2007 36:575-80. [Google Scholar]
[4]. Neville BW, Damm DD, Allen CM, Bouquot JE, Oral and Maxillofacial pathology 2002 2nd edPhiladelphiaW B Saunders:218-21. [Google Scholar]
[5]. Brouns ER, Baart JA, Bloemena E, Karagozoglu H, van der Waal I, The relevance of uniform reporting in oral leukoplakia: definition, certainty factor and staging based on experience with 275 patientsMed Oral Patol Oral Cir Bucal 2013 18(1):e19-26. [Google Scholar]
[6]. Schepman KP, Van der Meij EH, Smeele LE, Van der Waal I, Prevalence study of oral white lesions with special reference to a new definition of oral leukoplakiaEur J Cancer B Oral Oncol 1996 32B:416-19. [Google Scholar]
[7]. Mishra M, Mohanty J, Sengupta S, Tripathy S, Epidemiological and clinicopathological study of oral leukoplakiaIndian J Dermatol Venereol Leprol 2005 71:161-65. [Google Scholar]
[8]. Lee JJ, Hung HC, Cheng YG, Carcinoma and dysplasia in oral leukoplakias in Taiwan: prevalence and risk factorsOral Surg, Oral Med, Oral Path, Radiol and Endod 2006 101(4):472-80. [Google Scholar]
[9]. Chung CH, Yang YH, Wang TY, Shieh TY, Warnakulasuriya S, Oral precancerous disorders associated with areca quid chewing, smoking, and alcohol drinking in southern TaiwanJ Oral Pathol Med 2005 34(8):460-66. [Google Scholar]
[10]. Rodu B, Janssen C, Smokeless Tobacco and Oral Cancer: A Review of the Risks and DeterminantsCrit Rev Oral Biol Med 2004 15(5):252-63. [Google Scholar]
[11]. Freitas MD, Blanco-Carrión A, Gándara-Vila P, Antúnez-López J, García-García A, Gándara Rey JM, Clinicopathologic aspects of oral leukoplakia in smokers and nonsmokersOral Surg Oral Med Oral Pathol Oral Radiol Endod 2006 102(2):199-203. [Google Scholar]
[12]. Schepman K, Bezemer P, Van Der Meij E, Smeele L, Van Der Waal I, Tobacco usage in relation to the anatomical site of oral leukoplakiaOral Diseases 2001 7:25-27. [Google Scholar]
[13]. Hashibe M, Alcohol drinking, body mass index and the risk of oral leukoplakia in an Indian populationInt. J. Cancer 2000 88:129-34. [Google Scholar]
[14]. Axell T, Pindborg Shear M, International seminar on oral leukoplakia and associated lesions to tobacco habitsCommunity Dent Oral Epidemiol 1984 12:145-54. [Google Scholar]
[15]. Campisi G, Giovannelli L, Arico P, HPV DNA in clinically different variants of oral leukoplakia and lichen planusOral Surg Oral Med Oral Pathol Oral Radiol Endod 2004 98:705-11. [Google Scholar]
[16]. Martorell-Calatayud A, Botella-Estrada R, Bagán-Sebastián JV, Sanmartín-Jiménez O, Guillén-Baronaa C, Oral Leukoplakia: Clinical, Histopathologic, and Molecular Features and Therapeutic ApproachActas Dermosifiliogr 2009 100(8):669-84. [Google Scholar]
[17]. Prasanna Potentially malignant lesion – oral leukoplakia Glo Adv ResJ Med Med Sci 2012 1(11):286-91. [Google Scholar]
[18]. Kannan S, Expression of p53 in leukoplakia and squamous cell carcinoma of the oral mucosa: correlation with expression of Ki67J Clin Pathol: Mol Pathol 1996 49:M170-75. [Google Scholar]
[19]. Bailoor DN, Nagesh KS, Fundamentals of oral medicine and radiology. chapter 12 2005 First editionNew DelhiJaypee brothers medical publishers [Google Scholar]
[20]. Hansen LS, Olsen JA, Silverman S Jr, Proliferative verrucous leukoplakia. A long-term study of thirty patientsOral Surg Oral Med Oral Pathol 1985 60:5285-98. [Google Scholar]
[21]. Gandolfo S, Castellani R, Pentenero M, Proliferative verrucous leukoplakia: A potentially malignant disorder involving periodontal sitesJ Periodontol 2009 80:274-81. [Google Scholar]
[22]. Bagan JV, Proliferative verrucous leukoplakia: high incidence of gingival squamous cell carcinomaJ Oral Pathol Med 2003 32:379-82. [Google Scholar]
[23]. Ghazali N, Bakri MM, Zain RB, Aggressive, multifocal oral verrucous leukoplakia: Proliferative verrucous leukoplakia or not?J Oral Pathol Med 2003 32:383-92. [Google Scholar]
[24]. Barnes L, Eveson JW, Reichart PA, Sidransky D, World Health Organization classification of tumours. Pathology and genetics. Head and neck tumoursWorld Health Organization 2005 [Google Scholar]
[25]. Van der Waal I, Schepman KP, van der Meij EH, A modified classification and staging system for oral leukoplakiaOral Oncol 2000 36:264-66. [Google Scholar]
[26]. Garewal HS, Katz RV, Meyskens F, Pitcock J, Morse D, Friedman S, Beta-carotene produces sustained remissions in patients with oral leukoplakia: results of a multicenter prospective trialArch Otolaryngol Head Neck Surg 1999 125(12):1305-10. [Google Scholar]
[27]. Lodi G, Sardella A, Bez C, Demarosi F, Carrassi A, Systematic review of randomized trials for the treatment of oral leukoplakiaJ Dent Educ 2002 66(8):896-902. [Google Scholar]
[28]. Thomas G, Kunnambath R, Somanathan T, Mathew B, Pandey M, Rangaswamy S, Long term Outcome of Surgical Excision of Leukoplakia in a Screening Intervention Trial, Kerala, IndiaJ Indian Aca Oral Med Radiol 2012 24(2):126-29. [Google Scholar]
[29]. Chiniforush N, Kamali A, Shahabi S, Bassir SH, Leukoplakia removal by Co2 laserJ Lasers Med Sci 2012 3(1):33-35. [Google Scholar]