Clinical examination alone is considered inadequate to establish the diagnosis of leukoplakia because other oral white lesions may mimic leukoplakia [3]. Moreover, some leukoplakias may be associated with dysplastic changes which increase their likelihood of malignant transformation. Although, there has been a great amount of progress in the development of newer diagnostic methods [4–7] for diagnosis of oral cancer and precancer, biopsy and histopathological examination are still considered to be the gold standard.
The current study was undertaken to evaluate the usefulness of biopsy in assessing the clinico-pathologic correlations of oral leukoplakia at the patient’s initial visit.
Materials and Methods
Selection Criteria
The hospital records of patients with a clinical diagnosis of oral leukoplakia during the period from June 2001 to June 2007 were derived for this study. Lesions which were predominantly white in colour and could not be clinically diagnosed as any other disease of the oral mucosa were defined as leukoplakia lesions. All these patients with leukoplakia lesions had undergone biopsy in their initial visit and had signed a written informed consent.
Clinical Data and Evaluation
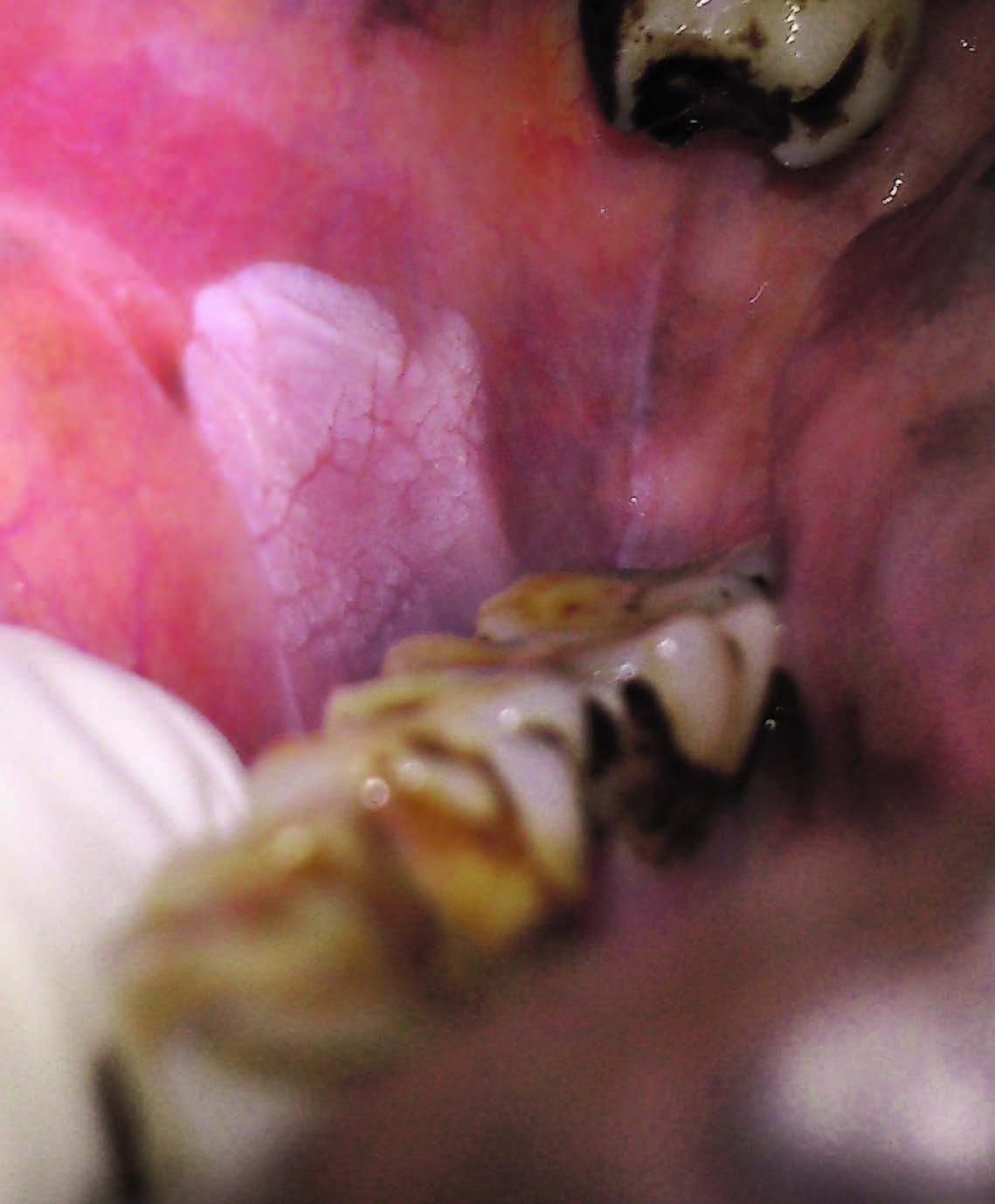
The data for analysis included the patient’s age, gender, oral habits and relevant medical history. Location, size and appearance of the lesion. Leukoplakia was defined as above and was classified as homogenous [Table/Fig-1], speckled/erythroplakia, nodular and verrucous leukoplakia [8]. Homogenous leukoplakia was considered when the patch was uniformly smooth or with a corrugated surface. Erythroleukoplakia was considered when the whitish lesion that included red areas. Whereas for nodular leukoplakia the lesion was slightly raised, rounded with, red and/or whitish excrescences that may be described as granules or nodules. Verrucous leukoplakia presents with finger like small projections in the white patch [8].
White lesion in the buccal mucosa suggestive of homogenous leukoplakia
Histopathology
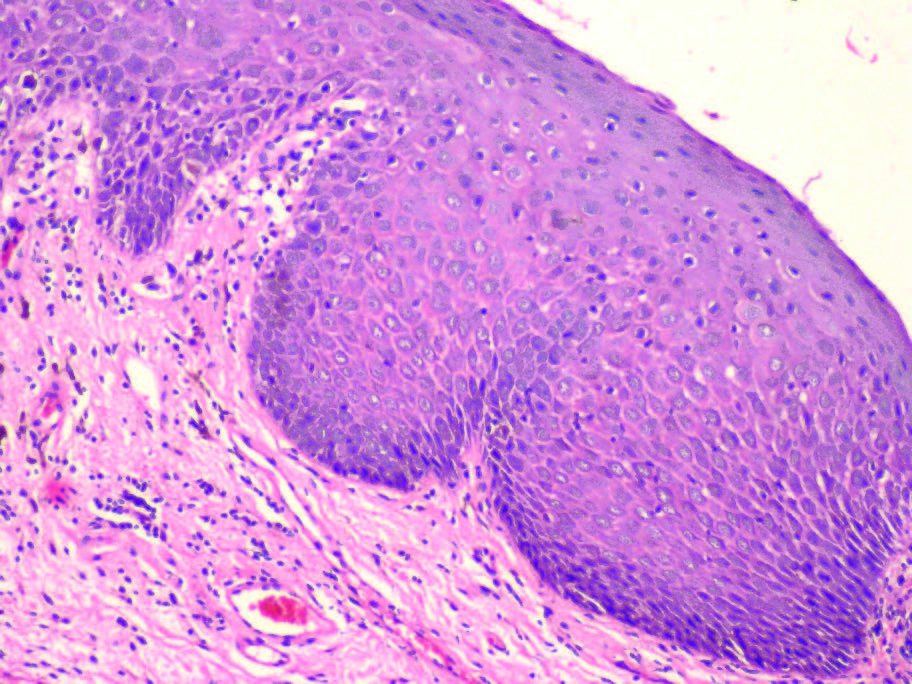
Standard protocols were followed for the preparation of these haematoxylin and eosin stained slides. Since the histopathological reports of the cases were initially reported by different pathologists, to ensure the uniformity of the histopathological diagnosis, a single pathologist reviewed all the slides to confirm the diagnosis and graded the presence of epithelial dysplasia. Histopathological diagnosis and grading of dysplasia were based on the WHO guidelines [9]. Lesions were categorized as Hyperkeratosis/acanthosis/verrucous hyperplasia with or without dysplasia (mild [Table/Fig-2], moderate or severe), squamous cell carcinoma (SCC) and verrucous carcinoma (VC).
Haematoxylin and Eosin stained section showing mild dysplasia with bulbous retepegs, basal cell hyperplasia and nuclear hyperchromatism (10X Magnification)
Statistical Analysis
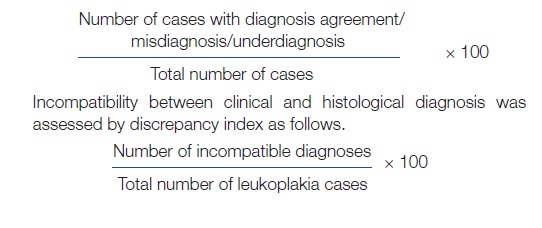
Diagnosis agreement was considered to be present when the clinical diagnosis (homogenous/speckled/verrucous leukoplakia) matched that of its histopathological diagnosis (hyperkeratosis/acanthosis/verrucous hyperplasia with or without dysplasia). Misdiagnosis was considered when the clinical diagnosis did not match its histopathological diagnosis (Example: a diagnosis of lichen planus in lesions clinically diagnosed as leukoplakia). Underdiagnosis was considered when malignancy was detected on histopathological examination.
The rates of diagnosis agreement, misdiagnosis and under diagnosis were calculated as:
Results
During the study period a total of 115 patients were clinically diagnosed with oral leukoplakia. The rate of incidence of leukoplakia was thus 19.16 cases per year. Of this total 107 were males and 8 were females (Male to female ratio of 13.37:1) [Table/Fig-3]. Majority of the patients were above the age of 40 year [Table/Fig-3]. [Table/Fig-4] shows the distribution of the types of leukoplakia in this sample.
| Sex Distribution | Age distribution (yrs) |
|---|
| Males | Females | 21-30 | 31-40 | 41-50 | 51-60 | 61-70 | 71-80 |
|---|
| 107 | 8 | 16 | 17 | 36 | 32 | 12 | 02 |
Distribution of different types of leukoplakia lesions and percentages of diagnosis agreement, misdiagnosis, underdiagnosis and dysplasia
| Clinical diagnosis | Total No. | Confirmation on H/P examination | Diagnosis agreement | Percentage of Misdiagnosis | No. of cases with dysplasia | Percentage of dysplasia | Number of cases with evidence of malignancy | Percentage of Under diagnosis |
|---|
| Homogenous Leukoplakia | 24 | 19 | 79.17% | 20.83 | 04 | 21.05 | 00 | - |
| Speckled Leukoplakia | 76 | 61 | 80.27% | 19.73 | 41 | 67.21 | 01 | 1.31 |
| Verrucous Leukoplakia | 15 | 08 | 53.33% | 46.67 | - | - | 07 | 46.67 |
| Oral Leukoplakia | 115 | 88 | 76.52% | 23.48 | 45 | 39.13 | 08 | 6.95 |
Histopathological examination confirmed the clinical diagnosis in 88 of the cases. The diagnosis agreement was thus 76.52% with a discrepancy index of 23.48%. Totally unrelated diagnoses were revealed in 19 cases, misdiagnosed in 16.52% of cases. Five of the 24 homogeneous leukoplakia cases were misdiagnosed with four of these proven histopathologically as lichen planus and one case as hyperplastic epithelium with no evidence of keratinization or acanthosis (not meeting the criteria for leukoplakia). As for the speckled leukoplakia cases, 14 of 76 cases were misdiagnosed. of these 11 cases were histopathologically diagnosed as lichen planus, two more cases as inflammatory epithelial hyperplasia and one case was verrucous hyperplasia. In eight cases with a clinical diagnosis of oral leukoplakia malignancy was detected upon histopathological examination. Underdiagnosis was thus present in 6.95% [Table/Fig-4].
Among the 24 patients with clinical diagnosis of homogeneous leukoplakia histopathological examination revealed a different diagnosis in five cases. The diagnosis agreement in the case of homogeneous leukoplakia was thus 79.16%. Among the remaining 19 cases correctly diagnosed as homogeneous leukoplakia, four (21.05%) cases showed histopathological evidence of dysplasia, but none showed malignancy [Table/Fig-4].
Among the 76 patients with clinical diagnosis of speckled leukoplakia histopathological examination revealed a different diagnosis in 14 cases and malignancy in one case. The diagnosis agreement was thus 80.27% and disagreement was 19.73%. The underdiagnosis was 1.13%. Among the 61 correctly diagnosed cases of speckled leukoplakia, 41(67.21%) showed dysplasia [Table/Fig-4].
Among the 15 patients with clinical diagnosis of verrucous leukoplakia histopathological examination confirmed clinical diagnosis in 8 patients and revealed malignancy in seven patients. The diagnosis agreement in verrucous leukoplakia was 53.33% and percentage of underdiagnosis was 46.67% [Table/Fig-4].
Discussion
Leukoplakia is a clinical term for a white lesion on the oral mucosa which cannot be diagnosed as any other clinical entity, i.e. it is a diagnosis of exclusion. Some of the other white lesions may clinically mimic oral leukoplakia and may be easily misdiagnosed as oral leukoplakia [3]. Historically many causative factors have been implicated in the pathogenesis of leukoplakia [10]. However, literature in the recent past has clearly associated leukoplakia with the use of tobacco or areca nut [3,11]. Lately studies have also focused on role of human papilloma virus [12]. Pathogenesis of leukoplakia has been thought to be characterized by impairment of the epithelial differentiation program [13]. Loss of heterozygosity, mutation of p53 gene, mitochondrial genomic mutations and epigenetic changes has been studied in leukoplakia which reflects it’s potentially malignant nature [14].
Although, there has been considerable progress in the development of diagnostic tools for the diagnosis of oral precancer including oral leukoplakia [4–6], biopsy and histopathological examination remain the gold standrad in their diagnosis. Some oral leukoplakias may be asymptomatic [15] and individuals with such lesions may be unaware of their presence. Convincing such patients to agree for biopsy may be a difficult task for the clinician.
The present study has looked into the usefulness of biopsy at the initial appointment in making a correct diagnosis of leukoplakia and in predicting its behavior.
During the study period of six years (June 2001 to June 2007) a total of 115 patients were diagnosed with oral leukoplakia. The rate of incidence of leukoplakia was 19.16 per year. This rate of incidence is higher compared to general population based studies where the annual incidence is about (0.6 – 8.9) per 1, 00,000 [16]. This could be due to a higher referral to the hospital being a tertiary centre. The geographic region of the study constitutes of a portion of rural population which cultivates and practices the use of tobacco, betel leaf and other products. This is also one of the reasons for higher incidence of tobacco associated lesions in this part of the world.
Majority of the patients with leukoplakia in the present study (71.13%) were above the age of 40 years. This is consistent with those of other studies [17] which have shown oral leukoplakia to affect persons above the age group of 40 year.
The male to female ratio in this study was 13.37:1. Several other studies have also shown leukoplakia to be predominantly affecting males [17,18]. This can be attributed to the increased indulgence of males in tobacco smoking and chewing habits.
Speckled leukoplakia was the most common type of oral leukoplakia in this study with 66.08% of the patients having this type of leukoplakia. Some of the other studies have shown homogeneous leukoplakia to be the most common type of clinical presentation [19,20].
In the current study dysplasia was seen in 39.13% of cases. In general, the frequency of epithelial dysplasia in leukoplakia varies between 1-30% [21–28]. However the lowest frequency has been reported in population based studies [24], which emphasizes the frequency of dysplasia. The presence of dysplasia in these lesions is one of the most important predictors of malignancy [29], which further stresses the need of early biopsy in all these lesions.
Epithelial dysplasia can be found in biopsies of the homogeneous leukoplakias but is more commonly diagnosed in non-homogeneous leukoplakias [29]. In the current study 4 of the 24 (21.05%) clinically diagnosed lesions of homogeneous leukoplakia had dysplasia. Some of the other studies have reported frequencies as high as 21% in these lesions [26]. Lee et al., reported a risk of less than 1% carcinomatous component in homogeneous leukoplakia lesions [30]. In contrast the homogeneous leukoplakia lesions in our study did not show any signs of malignancy.
Our study showed 41 of the 76 (67.21%) clinically diagnosed cases of speckled leukoplakia lesions with signs of dysplasia. Mishra et al., in their clinico-pathologic study on leukoplakia found dysplasia in 65.7% of speckled leukoplakia lesions [20]. The current study and previous studies reiterate the fact that a higher frequency of dysplasia should be anticipated in non-homogeneous lesions.
One of speckled leukoplakia lesions (1.31%) and 7 of the 15 (46.67%) verrucous leukoplakia lesions showed evidence of malignancy in the current study. Overall 6.95% lesions showed evidence of malignancy which is less than the percentage of malignancy detected in leukoplakia lesions by some of the other authors [18,23]. Waldron et al., in their landmark study on 3256 specimens of leukoplakia showed evidence of malignancy in 3.1% of the cases [23]. Wei Lu et al., in a recent study on 53 verrucous leukoplakia lesions found evidence of malignancy in 20.8% of cases [31]. Hence, it is not uncommon to anticipate evidence of malignancy in clinically diagnosed leukoplakia lesions. Therefore, it is advisable to biopsy these lesions at the initial appointment.
While most of the studies [18] have looked at the relation between the dysplasia and site of leukoplakia lesions the current study has looked at the relation between the dysplasia and clinical type of leukoplakia.
Conclusion
The authors are of the opinion that the clinical picture of leukoplakia lesions can be quite deceptive hence a biopsy at the initial appointment is strongly recommended. An early biopsy would provide much desired insight into the clinical severity of these lesions, Conversely a delay in the biopsy would also mean overlooking a frank malignant lesion or a severe dysplastic lesion.
“Part of the study was presented August 2012 FDI Annual World Dental Congress, Hongkong”