Chemoresistant Gestational Trophoblastic Neoplasia: A Case Report
Sudha CP1, Sahana M2
1 Associate Professor, Department of Obstetrics and Gynaecology, Kempegowda Institute of Medical sciences, Bangalore, India.
2 Post Graduate, Department of Obstetrics and Gynaecology, Kempegowda Institute of Medical sciences, Bangalore, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Sudha CP, Associate Professor, Department of Obstetrics and Gynaecology, KIMS Hospital, KR Road, VV Puram, Bangalore, KA, 560004, India.
Phone: 9035221739,
E-mail: cpdrsudha@gmail.com
Gestational trophoblastic neoplasia (GTN) is a disease of women in reproductive age. It is one of the most chemotherapy responsive and highly curable cancer. It is diagnosed when there is clinical, radiologic, pathologic, and/or hormonal evidence of persistent or relapsed gestational trophoblastic disease. In most instances, it is cured by surgical evacuation of the uterus. If persistent, it is treated with chemotherapy which provides response in >90% of the cases. In the unresponsive persistent cases and if the women has completed her child bearing, hysterectomy is generally recommended. Here, we report a rare case of chemoresistant GTN which was confirmed to be placental-site trophoblastic tumour (PSTT) on biopsy.
GTN, Placental site trophoblastic tumour, Uterine tumour
Case Report
In June 2011, a 24-year-old woman G3P1L1 (Gravida, Para, Abortion and Living), in her 4th month of her gestation, she visited a local hospital with history of passage of grape like mass and also had history of vaginal spotting in her first trimester. Ultrasonography (USG) done, showed bulky uterus with molar pregnancy. Her β hCG level was 2,31,450 mlU/ml. She underwent ultrasound guided suction evacuation. Her hemoglobin level was 6g/dl and she received three units of blood transfusion. Histopathology examination of specimen reported as molar pregnancy. On discharge, she was advised estimation of β hCG level at regular intervals. Patient was having regular menstruation after the evacuation.
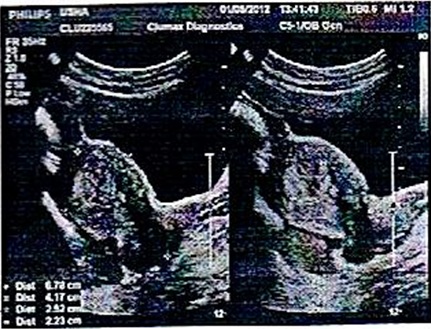
On 24th Feb. 2012, i.e. eight months following evacuation, the patient presented to kempegowda hospital Bangalore with history of vaginal bleeding for 13 days following regular cycles after evacuation and her last menstrual period was Feb 11th 2012, there was no evidence of pregnancy. On admission her vital status and general condition were poor showing severe degree of pallor, tachycardia (120/min), tachypnoea (32 breaths/min) and B.P of 90/50 mmHg. Her respiratory and cardiovascular examinations revealed no abnormality. On abdominal palpation, abdomen was soft, non tender and there was no organomegaly. Per speculum and bimanual examinations revealed congested cervix with mucoidal discharge, uterus soft in consistency, right fornix tenderness present. Her base line investigation showed Hb-7 gm%, TLC- 22,000/mm3, normal differential count, ABO/Rh- O+ve, urine R/E-NAD, S.TSH- 3.2 mIU/ml and total platelet count – 1.6 lakhs/ml. Blood sugar level, renal and liver function tests were all within normal limit. The striking rise of serum β hCG level to 11,203 mIU/ml was noted. USG scan showed a well defined hyperechoic lesion measuring 2.5*2.2*2 cm with few areas of heteroechogenecity in the center, the endometrium is splayed around the mass as seen in [Table/Fig-1] and MRI scan of abdomen-pelvis showed partial invasive mole in fundal region of the uterus. There was no evidence of metastases in other sites on the MRI, chest CT and brain CT. The patient was referred to gynaecologic oncologist.
Ultrasound guided scan shows well-defined hyperechoic lesion measuring 2.5 * 2.2*2 cm with few areas of heteroechogenecity in the center, the endometrium is splayed around the mass
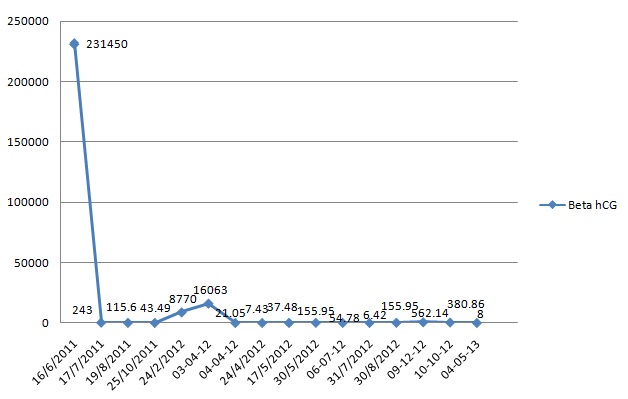
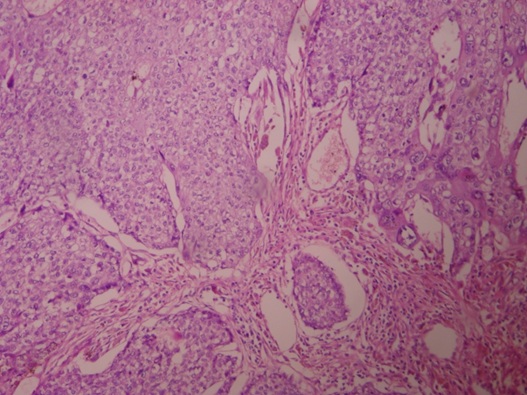
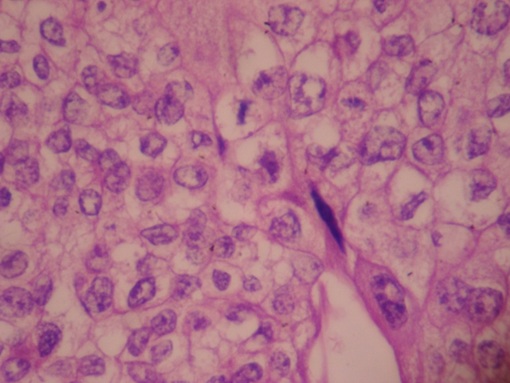
Patient was started on first line of chemotherapy with injection methotrexate 50 mg intramuscular weekly for total five cycles with folinic acid rescue at 21 days interval from March 2012 to May 2012. The β hCG levels were variable as shown in the [Table/Fig-2]. In June 2012, the patient was then started on second line chemotherapy with course of injection Dactinomycin, 12mcg/kg for five days. But even after six weeks course of chemotherapy, the hCG level was still high, so then patient was again put on with two cycles of chemotherapy with injection cyclophosphamide 600mg/m2 intravenously in saline over 30 minutes and injection Vincristine 1mg/m2 intravenously bolus over one minute given 21days interval. The β hCG levels were then subsequently estimated at the end of two months, which was 562.14 IU/ml, considered as high range. So, during the whole course of chemotherapy, there was a variable rise and fall of β hCG level as shown in [Table/Fig-2]. Ultimately, the patient was advised total abdominal hysterectomy on September 2012. The histopathology of the biopsy showed placental site trophoblastic tumour as described in [Table/Fig-3,4]. The patient was followed up for six months and she maintained negative β hCG levels.
Changes in β hCG levels (mIU/ml) during the treatment period
Microscopic pictures with 40x magnification and hematoxylin and eosin stain of section piece of mass showing large areas of hemorrhage and tumour cells infiltrating the 1/3rd of myometrium
Microscopic pictures with 400x magnification and hematoxylin and eosin stained tumour cells are arranged in sheets and groups, individual cells are large pleomorphic with abundant eosinophillic cytoplasmic pleomorphic vesicular nuclei
Discussion
Gestational Trophoblastic Neoplasms (GTN) are proliferative as well as degenerative disorders of placental elements and include complete or partial hydatidiform mole (90%), invasive mole (5-8%), villous or a villous choriocarcinoma (1-2%), and placental site tumour (1-2%) [1]. The incidence of GTN varies in different regions from 0.6 – 1.1 per 1,000 pregnancies in Europe and North America to 2 per 1000 pregnancies in Japan and 1 in 160 pregnancies in India and Middle East [2,3].
PSTT is the rarest form of GTN and originates from placental implantation site [4,5]. There are about 200 reported cases of PSTT all over the world [6]. It has infrequent occurrence and uncharacteristic clinical presentation. It has a wide clinical spectrum of presentation and behavior, ranging from a benign condition to an aggressive disease with a fatal outcome. PSTT is an extravillous infiltrating tumour that causes invasion of myometrium and mainly consists of intermediate trophoblasts. It was first described by Kurman et al., [7]. The term PSTT was coined by Scully and Young to describe its malignant potential [8]. PSTT may complicate or follow any type of normal or abnormal pregnancy (hydatiform mole) and its interval may vary from weeks to years after the preceding pregnancy. For patients with disease localized to the uterus, surgery remains the mainstay of therapy in the first instance, in contrast to other GTDs, because of its low chemosensitivity [9].
In our case, the patient was presented with symptoms of GTN that was diagnosed to be molar pregnancy by USG. Her β hCG level was high. The patient underwent suction evacuation but the β hCG level was still high and she was presented after eight months with vaginal bleeding. MRI scan of abdomen-pelvis showed partial invasive mole in fundal region of the uterus. The patient was started on chemotherapy with single agent first and then second line chemotherapy because of variable rise and fall in β hCG level. Ultimately, the patient was advised total abdominal hysterectomy. The HPE report was suggestive of PSTT. This explains the low chemosensitivity behaviour of the tumour. The report is presented here because of the challenges faced during the course of the treatment process and its rare occurrence following molar pregnancy.
Conclusion
One should emphasize on perusal of different treatment modalities to reduce the suffering and better outcomes for the patients.
[1]. Miller FM, Laing FC.Gestational trophoblastic disease http://brighamrad.harvard.edu/cases/bwh/hcache/34/full.html [Google Scholar]
[2]. Berkowitz RS, Goldstein DP, In: Berck JS. Gestational trophoblastic neoplasm 2002 PhiladelphiaLipincott, Williams and Wilkins:1353-74. [Google Scholar]
[3]. Chhabra S, Qureshi A, Gestational trophoblastic neoplasms with special reference to invasive moleObstet Gynecol India 2007 57(2):124-12. [Google Scholar]
[4]. John W L, Sarah E T, Faye F G, Robert P E, Placental site trophoblastic tumor: Immunohistochemistry algorithm key to diagnosis and review of literatureGynecol Oncol Case Rep 2014 7:13-15. [Google Scholar]
[5]. Whitney KA, Placental site trophoblastic tumourAm J Nurs. 2009 109(12):32-7. [Google Scholar]
[6]. Fengying H, Wenli Z, Qingchun L, Tuanfang Y, Diagnosis and treatment of placental site trophoblastic tumourInt J Clin Exp Pathol. 2013 6(7):1448-51. [Google Scholar]
[7]. Kurman RJ, Scully RE, Norris HJ, Trophoblastic pseudotumour of the uterus. An exaggerated form of Syncytial endometritis, simulating a malignant tumourCancer 1976 7:1214-26. [Google Scholar]
[8]. Scully RE, Young RG, Trophoblastic psuedotumour: A reappraisalAm J Surg Pathol. 1981 7:75-6. [Google Scholar]
[9]. Gupta Nupur, Sharma JB, Mittal Suneeta, Talwar Divya, Kumar Lalit, Kukreja Manu, Placental Site Trophoblastic Tumor of the Uterus: A Mistaken DiagnosisJK Science. 2010 12(1):31-32. [Google Scholar]