Pancreatic Schwannoma - A Rare Case Report
Devi J1, Sathyalakshmi R2, Chandramouleeswari K3, Nalli R. Sumitra Devi4
1 Post Graduate, Department of Pathology, Stanley Medical College, Chennai, India.
2 Assistant Professor, Department of Pathology, Stanley Medical College, Chennai, India.
3 Professor, Department of Pathology, Stanley Medical College, Chennai, India.
4 Associate Professor, Department of Pathology, Stanley Medical College, Chennai, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Devi.J, 66/2, Venkatrathinam Nagar, Adyar, Chennai-20, India. Phone : 09940663227, E-mail : devija@rediffmail.com
Pancreatic schwannomas are rare neoplasms. Only 47 cases have been reported in literature as of date. Age group that is commonly involved varies between the range of 20-87 years, with an almost equal sex ratio. Tumour size ranges from 1-20 cm. Locations where schwannomas can be encountered in the pancreas are in the vast majority, the head and body, the incidence being: head-40 %, junction of head and body-6 %, body-21 %, body and tail-15 %, tail-4 % and uncinate process-13 %, 60 % of the tumours are cystic, the rest being solid tumours. We hereby report a case where in total gastrectomy with distal pancreatectomy and splenectomy was done for carcinoma stomach involving the stomach bed and pancreatic schwannoma was an incidental finding in this case.
Antoni A and B areas, Distal pancreatectomy, Schwann cells, Splenectomy
Case Report
A 63-year-old female patient was admitted with complaints of abdominal pain, mass in the epigastric region with significant loss of weight for one year duration and endoscopy revealed an ulcerative lesion at the esophago-gastric junction. Imaging studies done showed the ulcerative lesion in the stomach with adhesion to the underlying structures and pancreas showed no significant lesion. She underwent total gastrectomy with distal pancreatectomy and splenectomy.
The total gastrectomy specimen received measured 20cm along the greater curvature and 14cm along the lesser curvature and showed an irregular, infiltrative, grey white lesion 4x1.5cm at the esophago-gastric junction. Distal end of pancreas measured 4x3x2.5cm and showed a tan yellow well defined nodule measuring 1x0.8cms,on cut section [Table/Fig-1]. Spleen appeared grossly normal.
Gross picture of distal end of pancreas with the yellow tan nodule measuring 1x0.8cms

Histopathological sections from the growth at esophago-gastric junction showed features of moderately differentiated adenocarcinoma infiltrating into the serosa.
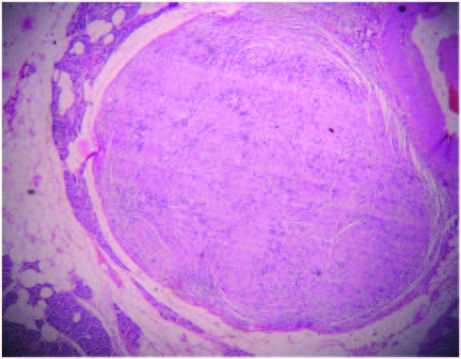
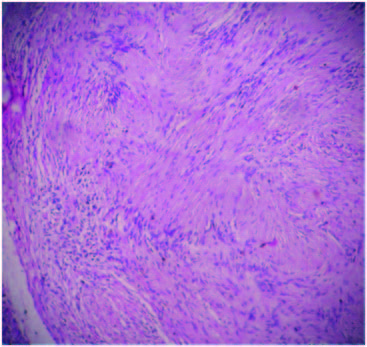
Sections from the nodule present in the pancreas showed an encapsulated neoplasm composed of hypercellular Antoni A areas with closely packed spindle shaped cells with wavy nuclei in palisading (Verocay bodies) and interlacing fashions and loose hypocellular Antoni B areas with thick walled blood vessels . A diagnosis of an incidental schwannoma was made. There was no evidence of tumour infiltration into the pancreas [Table/Fig-2,3]. Sections from the spleen showed features of congestive splenomegaly.
Scanner view: well encapsulated lesion in the pancreas
Antoni B and Antoni A areas with Verocay bodies 10 X view
Discussion
Verocay reported schwannoma as a true neoplasm that originated from the schwann cells in 1910 [1]. The anatomic distribution of schwannomas is very wide with majority of cases arising in the extremities. Other sites include trunk, head and neck, retroperitoneum, mediastinum, cranial nerves, bone, gastrointestinal tract, subcutaneous tissue and muscle. Schwannomatosis (multiple peripherally located schwannomas) are associated with SMARCB 1 mutation and inactivation of NF2. Pancreatic schwannomas are rare neoplasms that originate from the Schwann cells. Pancreatic schwannomas arise from the branches of the vagus nerve which courses through the pancreas [2–4]. Symptomatic patients present with abdominal pain, backache, nausea, vomiting, weight loss, melena and jaundice. In asymptomatic patients, the lesions may be incidentally found.
Classic schwannomas are encapsulated neoplasms composed of hypercellular and hypocellular areas. Hypercellular or the Antoni A area consists of monomorphic spindle shaped schwann cells, composed of poorly defined eosinophilic cytoplasm and pointed basophilic nuclei, in a variably collagenous stroma. These cells in the Antoni A areas show nuclear palisading, and parallel arrays of such palisades with intervening cell processes are called Verocay bodies. Antoni B areas or hypocellular areas are composed of schwann cells with inconspicuous cytoplasm and nuclei appearing to be suspended in a copious myxoid, often microcystic matrix. Degenerative changes like hyalinisation, cystic changes, xanthomatous calcification or hemorrhage are often recognised in the Antoni B areas [5]. These degenerative changes are due to vascular thrombosis and necrosis [6]. Normally, less than five mitotic figures per 10 high power fields can be seen in a benign schwannoma. Of note is that schwannomas arising in the gastrointestinal tract or upper respiratory tracts are unencapsulated, and those located in the gastrointestinal tract are surrounded by a prominent peripheral lymphoid cuff. Very rarely, pancreatic schwannomas can undergo malignant transformation.
Immunohistochemically, pancreatic schwannomas are positive for S 100, Vimentin and CD 56. Ultrastructurally, benign schwannomas are composed of cells with small cell bodies and elongated, interdigitating cytoplasmic processes invested by a complete external lamina. These processes are connected by desmosome like junctions. Cytogenetic analysis studies show that most schwannomas show either monosomy 22 or loss of 22q material.
Cystic pancreatic schwannomas can mimic intraductal mucinous papillary neoplasms, mucinous cystic neoplasms, serous cystic neoplasms, solid pseudopapillary neoplasms, lymphangiomas and pancreatic pseudocysts.
Pre-operative diagnosis of pancreatic schwannomas is difficult even with recent imaging modalities [7]. The final diagnosis of these Tumours is made only after operative excision and histological studies. A variety of imaging modalities like Ultrasound, CT scan and MRI helps in narrowing the differentials. The tumours are usually well defined, hypoechoic with encapsulation and /or cystic degeneration on CT [8–10]. Tumours with high Antoni A areas appear inhomogenous due to increased lipid content and that of Antoni B areas appear cystic and multiseptated and show low density [8,10] due to loose stroma and low cellularity. With contrast Antoni A areas show enhancement due to the increased vascularity and the Antoni B areas are non enhancing due to less vascularity [8,10]. MRI outlines the degree of vascular involvement of these tumours and is of great help in assessing the potential biological behavior of these tumours as benign or malignant [9–11]. On T1 weighted images the pancreatic schwanommas appear hypointense and hyperintense in T2 weighted images [9–11]. Solid components of the tumour are well appreciated by ultrasonography than CT and MRI. However, all these radiological features are not specific for pancreatic schwanommas and can be noticed in other pancreatic cystic lesions [8]. The effectiveness of ultrasound guided FNA in these tumours is questionable due to insufficient specimen collection and defects in collection technique [12]. Cytologically, schwannomas are composed of spindle cells with indistinct cytoplasmic borders and wavy nuclei embedded in a fibrillary and occasionally myxoid or collagenous matrix [13,14].
Two thirds of pancreatic schwanommas are cystic and could be mistaken for cystic neoplasms of pancreas either benign or malignant. Establishing the diagnosis prior to surgery is imperative because the treatment of a benign schwanomma of the pancreas is enucleation as compared to radical surgery in case of malignant lesions. Intraoperative frozen section is essential in all pancreatic schwanommas with complete histopathological and immunohistochemical examination for accurate diagnosis and to avoid unnecessary radical resections for benign lesions [15].
Conclusion
Pancreatic schwannoma is an important entity to be included in the differential diagnosis of pancreatic lesions. This non epithelial non endocrine pancreatic tumour should not be neglected because of its biologic and prognostic significance. For benign lesions, simple enucleation is adequate, whereas malignant tumours require standard oncological resection.
[1]. Verocay J, Zur Kentnis der “Neurofibrome”Beitr Pathol Anat Allg Pathol 1910 48:1-69. [Google Scholar]
[2]. Almo KM, Traveso LW, Pancreatic schwannoma: an uncommon but important entityJ Gasterointest Surg. 2001 5:359-63. [Google Scholar]
[3]. David S, Barkin JS, Pancreatic schwannomaPancreas. 1993 8:274-76. [Google Scholar]
[4]. Paraiyapa C, Johnson SR, Khwaja K, Goldman H, Kruskal JB, Hanto DW, Clinical characteristics, treatment and outcome of pancreatic schwannomasJ Gasterointest Surg 2004 8:706-12. [Google Scholar]
[5]. Enzinger FM, Weiss SW, Benign Tumours of the peripheral nerves in Soft Tissue Tumours, F.M.Enzinger,Ed., pp 821-828 1995 3rd EditionSt.Louis,Mo, USAElseiver Saunders [Google Scholar]
[6]. Weiss SW, Langloss JM, Enzinger FM, Value of S 100 protein in the diagnosis of soft tissue tumours with particular reference to benign and malignant Schwann cell tumoursLab invest. 1983 49:299-308. [Google Scholar]
[7]. Ahmed Abu-Zaid, Ayuman Azzam, Hussam Abou Al-Shaar, Abdullah M Alshammari, Tarek Amin, Shamayel Mohammed, Panceatic Tail Schwanomma in a 44 year old male: A Case Report and Literature ReviewCase Reports in Onological Medicine 2013 2013(2013)Article ID 416713,5 pages [Google Scholar]
[8]. Suzuki S, Kaji S, Koike N, Pancretic Schwanomma: A Case Report and Literature Review with special references to imaging featuresJournal of the Pancreas 2010 11(1):31-35. [Google Scholar]
[9]. Feldman L, Philpolts LE, Reinhold C, Duguid WP, Rosenberg L, Pancreatic Schwanomma: Report of two cases and review of literaturePancreas. 1997 15(1):99-105. [Google Scholar]
[10]. Ferrozzi F, Bova D, Garlaschi G, Pancreatic Schwanomma: Report of three casesClinical Radiology 1995 50(7):492-95. [Google Scholar]
[11]. Novellas S, Chevallier P, Paul MC Saint, Gugenheim J, Bruneton JN, Magnetic Resonance Imaging features of a pancreatic schwanommaCliical Imaging. 2005 29(6):434-36. [Google Scholar]
[12]. Gupta PK, Baloch Z, Sack MJ, Yu GH, Difficulties in the FNA diagnosis of schwanommaCytopathology 1999 10:186-94. [Google Scholar]
[13]. Li S, Ai SZ, Owens C, Kulesza P, Intrapancreatic schwannoma diagnosed by endoscopic ultrasound-guided fine needle aspiration cytologyDiagn. Cytopathol 2009 37:132-35. [Google Scholar]
[14]. Hirabayashi K, Yasuda M, Umemura S, Itoli H, Itoli J, Yazawa N, Cytological features of the cystic fluid of pancreatic schwannoma with cystic degenerationA case report J OP 2008 9:203-8. [Google Scholar]
[15]. Moriya T, Kimura W, Hirai I, Pancreatic schwanomma: Case Report and an Updated 30 years review of the literature yielding 47 casesWorld Journal of Gastroenterology 2012 18(13):1538-44. [Google Scholar]