Diabetes mellitus (DM) is a complex multi-systemic metabolic disorder characterized by a relative or absolute insufficiency of insulin secretion and/or concomitant resistance to metabolic action of insulin on target tissues [1]. According to the World Health Organization (WHO) criteria, in India the prevalence of known diabetics was 5.6% and 2.7% among urban and rural areas respectively, [2] with incidence of nearly 2% thus earning it the dubious distinction of being termed the “Diabetes capital of the world” [3]. The effects of this disease on saliva and its constituents modulate the oral ecology, which have an important role to play in the cause and progress of mucosal lesions. The prevalence of oral candidal infections among diabetics in the current study is consistent with numerous previous studies [4], which have shown that DM is a major predisposing factor to symptomatic candidosis, oral or otherwise, but there is a paucity of data on the compostition of saliva and candidal status in type-2 diabetes mellitus. Salivary changes offer interesting parameters to evaluate and monitor diabetes mellitus and has opened up new vistas of research. Saliva is inexpensive, noninvasive and less cumbersome screening method as compared to serum and urine in terms of collection, storage and voluminous sampling. Its noninvasive nature increases patients’ willingness to continue health related examinations over time [5].These reasons prompt an interest in evaluating the possibility of using saliva as a diagnostic resource. The aim of this study was to detect salivary glucose levels in diabetic and non-diabetic subjects, to study the relationship between salivary glucose levels and oral candidal carriage. Also to evaluate if salivary glucose levels may then be used as a quick and cost effective routine investigation to indirectly assess the oral candidal carriage so as to help advice patients about precaution to maintain good oral hygiene and strict diabetes control to prevent clinically overt candidiasis and its related morbidity.
Methods
The study population included type-2 diabetic patients and sex and age matched non diabetic controls. The subjects included in this study were selected from Outpatient Department of Bapuji Dental College and Hospital, Davangere Karnataka, India. Non-stimulated whole saliva was analysed in this study. The subjects were divided into 2 Groups: Group I (Type-2 diabetic patients: 30 cases) composed of patients 30-60-year-old and Group II (non-diabetic subjects; 30 cases). Random non fasting plasma glucose (RNFPG) levels were performed in duplicate, once as a part of routine follow-up and again one week later.
Diagnostic Criteria for Diabetes
All diabetic patients in this study had been diagnosed for diabetes using established criteria [6]. Patients with severe diabetic complications were excluded. Study participants with any other systemic illness or on medications other than for diabetes were avoided.
Saliva Collection
The saliva collection was standardized as far as possible. Unstimulated whole saliva was taken for the estimation of mixed salivary glucose (MSG) levels as stimulation may alter the salivary composition [7]. Salivary sample collection was done in the morning between 8.00-11.00 a.m. with study subjects sitting upright in a comfortable position in calm isolated room. Study subjects were instructed not to smoke or brush or eat or drink two hours prior to the time of saliva collection. In the beginning, subjects were asked to spit out or swallow whatever saliva was present in the mouth and the samples collected for initial 30sec were discarded. Salivary samples were collected in the ice-chilled graduated saliva collector by spitting method. Saliva collection was done at least for five minutes. One ml of each unstimulated salivary sample was centrifuged at 3000 rotations per minute for 20 minutes and soon the clear supernatant were processed for estimation of salivary glucose.
Measurement of Mixed Salivary Glucose Levels
Glucose levels of unstimulated saliva were measured using the glucose oxidase method in a semiautomated biochemical analyser. 1000 µl of reagent solution was pipetted in each of the two test tubes. Ten µl of standard was added to one and 10 µl of salivary test sample was added in the other and mixed well. Thousand µl of blank reagent was aspirated in the analyser, followed by the standard solution and lastly, the test sample and incubated for 5 min at 37°C. The absorbance values of standard and the sample against the reagent blank was measured at 505 nm.
Microbiologic investigations: Phosphate buffer saline was used for oral rinse sampling in this study as it is known to be a sensitive technique for estimating the oral candidal carriage [8]. Each subject was requested to rinse the mouth thoroughly with 10 ml of sterile phosphate buffer saline (PBS, pH 7.2, 0.1 mol/l) for 60sec and expectorate the rinse into a sterile universal container. The supernatant was discarded and 0.001mL inoculating loop was used to spread the sample onto Sabouraud dextrose agar plates supplemented with chloramphenicol (10 mg/mL), then incubated aerobically at 37oC for 48h. Candida cfu/ml was calculated on each plate by digital colony counter. Candida albicans were identified as those with germ tube formation in serum [9]. Germ tube test is done by inoculating a small portion of a pure colony grown on plates into sterile test tubes containing 0.5ml of each of the test sera with a sterile platinum loop. The serum culture was incubated at 370C for 2-3 hours, but no longer than 3h, as other species of yeasts will form germ tubes with longer incubation times [9].
These were studied with two stains: Periodic acid schiff stain and Grocott-Gomori stain.
Ethics: The written consent for candidal carriage investigations, collection of saliva and blood for glucose estimation to be carried out in the study were obtained from reporting subjects after the necessary instructions and the study was approved by the ethical committee of the institution.
Statistical Analysis
T-test was used for comparing means of two Groups. Chi-square test was used for analysing categorical data such as the prevalence of candidal carriage in two Groups. Spearman’s correlation coefficient was used for measuring relationship between salivary glucose with colony forming units (CFU). P-value of 0.05 or less was considered for statistical significance.
Results
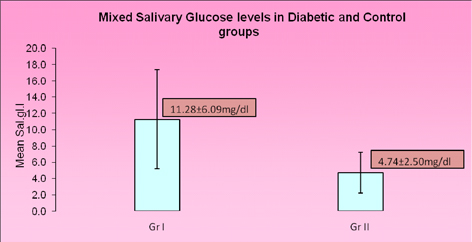
Mixed salivary glucose concentration in diabetics (11.28±6.09 mg/dL) was significantly higher compared to the controls (4.74±2.50 mg/dL) (statistical analysis with unpaired t-test showed p<0.01, t=5.44) [Table/Fig-1].
Mixed salivary glucose levels in Diabetics (Group I) and Controls (Group II)
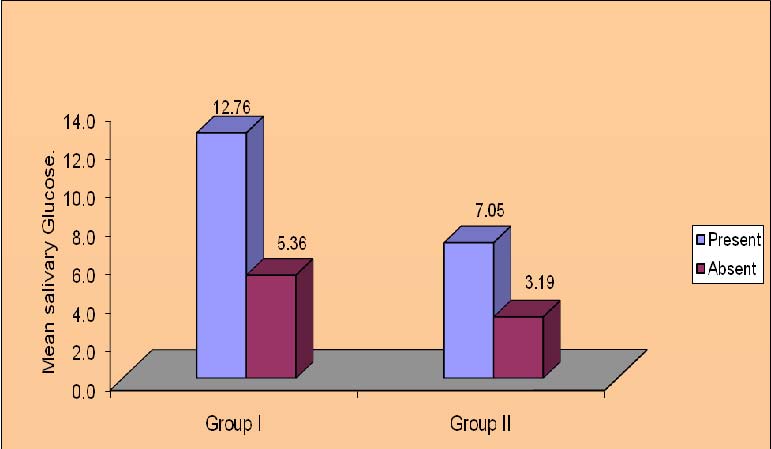
The significant finding of the present study is that diabetics with intraoral candidal carriage had higher MSG levels (mean = 12.76±5.85 mg/dl) compared to cases where Candida was not isolated. The diabetics without intraoral candidal carriage had lower salivary glucose levels (mean = 5.36 ± 2.24 mg/dl). This relationship could be seen in controls (nondiabetics) also. Nondiabetics who showed the presence of Candida in oral cavity, had higher salivary glucose levels (mean = 7.05±2.24 mg/dl) as compared to controls who did not show the presence of Candida in oral cavity(3.19±1.05 mg/dl). There was a significant relationship between the candidal prevalence and MSG levels in Group I( p<0.01, t=3.01) and Group II( p<0.01, t=6.37). Thus suggesting that, higher MSG levels showed the presence of Candida in the oral cavity compared to lower MSG levels [Table/Fig-2].
Mixed salivary glucose levels in relation to candidal carriage in Diabetics (Group I) and Controls (Group II)
Non significant correlation was detected between duration of diabetes and candidal status with Chi-square test (p=0.91 and x2=0.18) [Table/Fig-3]. In both the Groups the increase in salivary glucose levels did not show increase in CFU on Sabouraud dextrose agar plates. The quantity of candidal growth was determined by the number of colony forming units per ml (CFU/ml) in patients isolated with Candida species. A non-significant p-value of 0.70 in Group I and 0.39 in Group II was obtained [Table/Fig-4].
Duration of diabetes and candidal status in Group I
| Duration of diabetes | No of cases | Candida present | Percentage |
|---|
| ≤ 1 years | 5 | 4 | 80% |
| 2-5 years | 17 | 14 | 82.4% |
| ≥ 6 years | 8 | 6 | 75.0% |
| Total | 30 | 24 | 80% |
Correlation between mixed salivary glucose levels and CFU/ml (colony forming units/ml) in Candida positive subjects in Group I and Group II
| Relationship between salivary glucose and Colony forming unit | Group I | Group II |
|---|
| r-value | p-value | r-value | p-value |
|---|
| 0.10 | 0.70 | 0.27 | 0.39 |
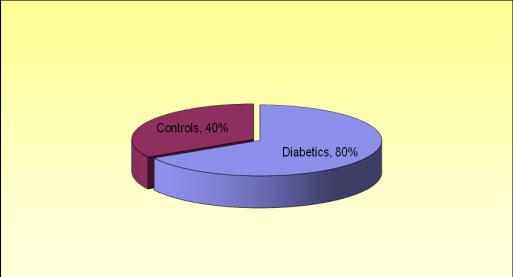
Diabetics showed an oral candidal carriage rate of 80% which was significantly higher compared to non-diabetics who showed an oral candidal carriage rate of 40%. This shows that prevalence of candidal carriage was significantly higher in diabetics compared to controls(x2= 10.0; p=.002; [Table/Fig-5].
Prevalence of candidal carriage in Diabetics and Controls
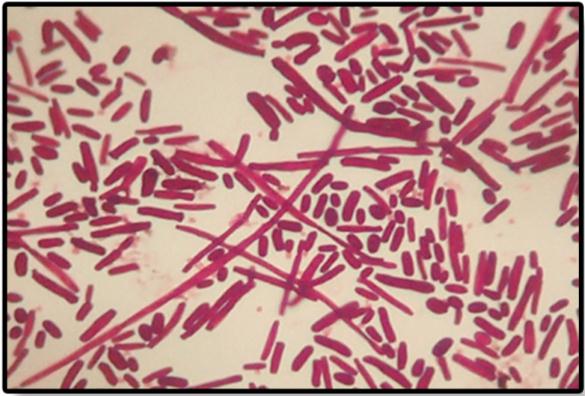
In diabetics 82.3% of Candida species isolated showed germ tube formation. In controls, 75% of Candida species isolated showed germ tube formation. Thus, indicating that majority of Candida species isolated were Candida albicans.
Discussion
Saliva plays a critical role in maintenance of oral and dental health. Knowledge of the components of saliva is important because they may indicate oral or systemic alterations, and it is also important because saliva may be a substitute for blood in lab tests for the diagnosis of illnesses [10]. Diabetes has been one of the most frequently blamed as a risk agent for dental, gingival inflammation and salivary alterations. In this study, salivary glucose was measured in unstimulated saliva of both type-2 diabetics and controls. The results in the present study differed from those of other studies [4,11]. One explanation for these differences may be the choice of certain study designs, as well as the diversity of the methods, dietary pattern and criteria for selecting the samples. However, results in the present study were nearly similar to the measurement of 5.94 mg/dl for the non-diabetics as found in the study done by Soares et al., [10]. The significant finding of this study was increase in MSG level in diabetics(11.28±6.09 mg/dl) compared to non-diabetics (4.74±2.50 mg/dl) which was observed by other authors also [4,11–13]. It has been suggested that the low molecular weight of glucose enables it to diffuse readily through the semi-permeable membrane. As in diabetics the basement membrane of parotid salivary gland is more permeable, so elevated blood sugar levels may cause increase in salivary glucose levels [7]. Although a threshold mechanism for salivary glucose has been suggested in humans, its nature and detailed information on the mechanisms involved have yet to be clarified. Until detailed information is available on the many possible influences on MSG levels it is clear that salivary glucose cannot as yet be used as an alternative to blood for monitoring glycaemic control in diabetic patients.
The prevalence of oral candidal carriage in type-2 diabetes mellitus in the current study is consistent with numerous previous studies [4,8,11]. There was a significant positive correlation between salivary glucose and oral candidal carriage in the overall study population, confirming the observation by other investigators that oral candidal carriage reflects increased salivary glucose levels.
In our study subjects, Group I and Group II were considered for the presence of Candida in oral cavity only after confirming with Periodic acid-Schiff stain and GrocottGomori stain and was not dependant on CFU since the growth seen in the culture plates may be due to other microorganisms also. Both these stains are specific for the identification of fungal organisms.
Staining of organisms showed the presence of spores and hyphae formation [Table/Fig-6]. Candida albicans were identified as those with germ tube formation [Table/Fig-7]. Over 90% of Candida albicans isolated from clinical material produces germ tube formation when incubated in serum at 370C. Other yeasts generally do not form germ tubes within these three hour time frame [9,14]. Therefore, germ tube test is a rapid presumptive test for the identification of Candida albicans [15]. Staining by PAS stain & GMS stain revealed 80% positive staining for Candida organisms in diabetics which were significant compared to controls i.e. 40%.
Photomicrograph of Periodic acid-Schiff stain showing the presence of spores and hyphae formation
Photomicrograph of Grocott-Gomori stain showing germ tube formation
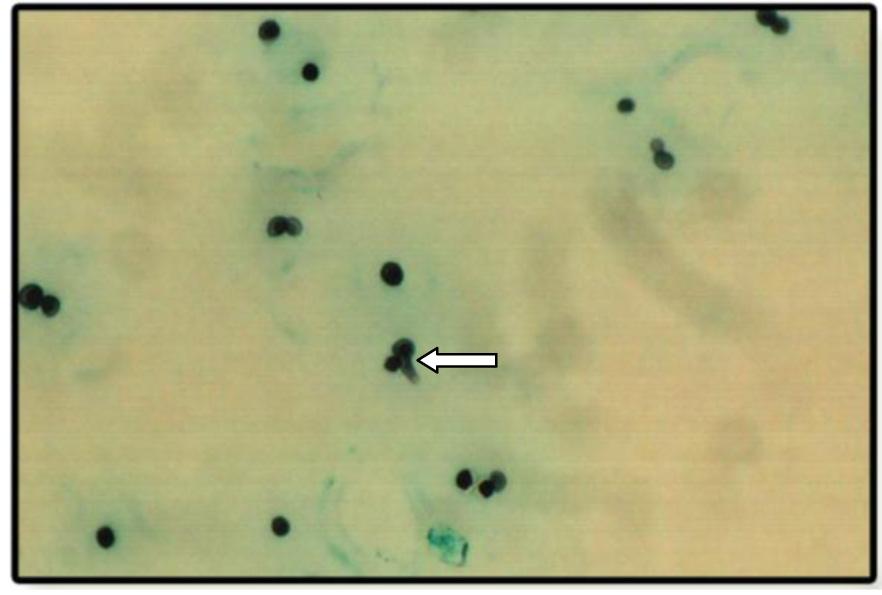
Several reasons for the high oral candidal carriage in diabetics have been suggested. One possible mechanism that has been proposed is that the adhesion of Candida to buccal epithelial cells of diabetic patients is significantly greater implying that there are intrinsic qualitative changes in the cell-surface receptors of diabetics that modulate yeast adhesion [16]. It has also been suggested that salivary glucose forms chemically reversible glycosylation products with proteins in tissues during hyperglycemic episodes, this leads to accumulation of glycosylation products on buccal epithelial cells, which in turn may increase the number of available receptors for Candida [9]. Also, candidal growth in glucose supplemented saliva has been shown to promote acid production, lower pH and enhance candidal proteolytic activity. Intraoral acidic pH conditions, in turn activate the production of candidal extracellular phospholipases [4].Other factors contributing to the pathogenesis of candidiasis are the candidacidal activity of neutrophils which may be defective, particularly in the presence of glucose, a further mechanism that may aid candidal infestation in diabetics [17] and decreased salivary flow rate which also reduces the protective function of saliva [9].
Non-significant correlation was detected between duration of diabetes and candidal status. There was no significant relationship between the quantity of candidal growth and mixed salivary glucose levels in both diabetics and non-diabetics. Whether, this reflects the sensitivity of the test used or other factors need further investigation. The invitro study of Knight & Fletcher showed a correlation between the quantity of candidal growth and glucose levels in saliva from the diabetic patients [18] but in our study this relation could not be confirmed which agree with other earlier studies. It is however difficult to extrapolate in-vitro results to in-vivo situation since many factors can affect the candidal growth in saliva and not all of these are reproducible in-vitro.
Conclusion
The present findings show that mixed salivary glucose levels were significantly higher in diabetics compared to controls. Diabetics with intraoral candidal carriage had significantly increased salivary glucose levels. Salivary glucose levels can therefore be used as a quick, non-invasive and cost effective routine investigation to indirectly assess the oral candidal carriage in diabetics so as to help advice patients to maintain good oral hygiene and strict diabetes control to prevent clinically overt candidiasis and its related morbidity.