A Rare Variant of Wallenberg’s Syndrome: Opalski syndrome
Parathan KK1, Kannan R2, Chitrambalam P3, Senthil Kumar Aiyappan4, Deepthi N5
1Assistant Professor, Department of General Medicine, Saveetha Medical College & Hospital, Saveetha University, Saveetha Nagar, Thandalam, Chennai, Tamilnadu, India.
2Associate Professor, Department of General Medicine, Saveetha Medical College & Hospital, Saveetha University, Saveetha Nagar, Thandalam, Chennai, Tamilnadu, India.
3Professor, Department of General Medicine, Saveetha Medical College & Hospital, Saveetha University, Saveetha Nagar, Thandalam, Chennai, Tamilnadu, India.
4Associate Professor, Department of Radiodiagnosis and Imaging, Saveetha Medical College & Hospital, Saveetha University, Saveetha Nagar, Thandalam, Chennai, Tamilnadu, India.
5Junior Resident, Department of General Medicine, Saveetha Medical College & Hospital, Saveetha University, Saveetha Nagar, Thandalam, Chennai, Tamilnadu, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Kannan R, Associate Professor, Department of General Medicine, Saveetha Medical College & Hospital, Saveetha University, Saveetha Nagar, Thandalam, Chennai – 602 105, Tamilnadu, India.
Phone: 9710071284,
E-mail: nalini.kadgi@gmail.com
Lateral Medullary Syndrome (LMS) is a well-documented vascular syndrome of the posterior circulation territory. This syndrome is easily localised because of characteristic presentation, unique territory of blood supply and very small area of involvement. We present a case of Wallenberg’s syndrome which did not have all the classical components of the syndrome, like Horner’s syndrome. Opalski syndrome is a rare variant of Wallenberg syndrome, where lateral medullary syndrome is associated with ipsilateral hemiparesis. This case report highlights how differential involvement of the lateral part of medulla can result in varied presentation.
Hemiparesis, Lateral Medullary Syndrome, Opalski syndrome, Wallenberg’ s syndrome
Case Report
A 65-year-old gentleman presented to our hospital with history of sudden onset weakness of right upper and lower limb, numbness of the left half of his body and limbs, burning sensation over right half of face, and gait instability with multiple episodes of fall, always to his right side. He gave history of hoarseness of voice but there was no nasal regurgitation. He was neither a known diabetic or hypertensive, never had hypothyrodism or dyslipidaemia earlier. There was no history of trauma in the past. He was not a smoker but had history of chronic alcohol consumption with history of an alcohol ‘binge’ preceding the onset of his current symptoms.
General examination, vital parameters, cardiovascular, respiratory and abdominal examination were unremarkable. On detailed neurological examination higher motor functions were normal. Cranial nerve examination revealed dysaesthesia over right half of the face and right side lower facial weakness (right VII nerve UMN palsy). Motor system examination revealed right side hemiparesis with extensor plantar reflex. Sensory system examination showed decreased perception of pain and temperature over the left half of his trunk and limbs. He also had truncal ataxia. There was no evidence of Horner’s syndrome.
Routine blood profile was within normal limits except for haemoglobin of 16.3 g/dL and PCV of 50.2%. Blood sugars, lipid profile, thyroid profile, liver function, renal function, chest radiograph, ECG and Echocardiogram were within normal limits. Screening test for both HIV and syphilis were negative.
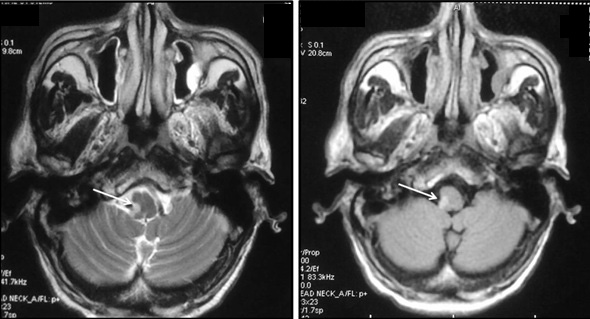
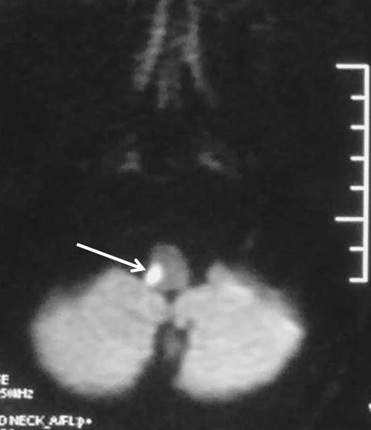
Magnetic resonance imaging (MRI) brain revealed an infarct in the dorsolateral aspect of the Right side of medulla consistent with lateral medullary syndrome [Table/Fig-1,2]. Magnetic resonance angiogram did not reveal any occlusion in the vertebral, basilar and cerebral arteries.
The condition of the patient improved within few days with conservative line of treatment like anti-platelets, vitamins and physiotherapy. Neurological examination at discharge revealed only mild impairment of sensation over the left half of his body and facial pain over the right half of his face. Follow up at one month after discharge revealed persistence of mild sensory deficit but patient was able to lead a fully independent life.
Axial T2-weighted and FLAIR MRI images showing small focal area of hyperintensity involving dorsolateral medulla on right side (white arrows) suggestive of infarct
Axial diffusion weighted MRI image showing focal area of diffusion restriction in dorsolateral medulla on right side (white arrow) suggestive of acute infarct
Discussion
Lateral Medullary Syndrome (LMS) was described by Wallenberg [1]. It is a relatively uncommon stroke with slight male sex preponderance [1,2]. The classical presentation consists of crossed sensory deficits, specifically loss of pain and temperature sensation affecting trunk and extremities contralateral to the infarct along with ipsilateral facial numbness. Other features of the syndrome include vertigo, nystagmus, hoarseness, dysphagia, ipsilateral cerebellar signs and Horner’s syndrome [2].
In most cases the diagnosis of LMS is made on the basis of classical clinical features and some authors even recommend delaying the MRI scan when the presentation is classical [3]. However, a wide variation has been recognised in the clinical presentation [3,4] and studies have demonstrated that the classical presentation is not seen most of the time. Hence, efforts have been made to classify LMS on the basis of pattern of sensory involvement [5], anatomical localisation [6] and radiological pattern of involvement [1]. Our patient’s presentation differed from the classical description of Wallenberg in several aspects. Although, he had classical crossed sensory involvement; he had neither nystagmus nor Horner’s syndrome. He had additional features like facial pain and contralateral hemiparesis. We shall examine each of these aspects in detail.
The wide variation in sensory involvement has long been recognised
and Zhang et al., described five patterns of sensory impairment [5].
Type 1: Ipsilateral face and contralateral trunk and limbs
Type 2: Ipsilateral face and contralateral face, trunk and limbs
Type 3: Contralateral face and body
Type 4: Ipsilateral face and contralateral trunk and leg
Type 5: Contralateral face, arm and upper trunk
Our patient would come under classical or type 1 sensory involvement, as he presented with right sided face and left sided trunk and limb involvement. In clinico radiological correlation studies by Kim et al., only 26% of the patients presenting with the classical crossed sensory pattern or Zhang type 1 and 25% presented with Zhang type 2.This variation has been attributed to the different patterns of involvement of medulla classified horizontally as dorsal, ventral and lateral and vertically as rostral and caudal [1]. The more rostral lesions which tend to be more ventral presented with contralateral trigeminal nerve involvement while the more caudal and dorsolateral lesions tend to present classically as seen in our case [3].
The absence of the classical component of Wallenberg’s syndrome – Horner’s syndrome is also explained by this differential involvement. Kim et al., have demonstrated that Horner’s syndrome is uncommon when the dorsal part of medulla is involved, as seen in our patient [1]. It was also observed that dorsolateral lesions tend to be more superficial and hence do not involve nucleus ambiguous, which is situated more deeply [1]. This explains the absence of dysphagia and signs of palatal paralysis in our patient. The spontaneous facial pain experienced by our patient is part of some of the original descriptions of Wallenberg syndrome. It has been described as a burning type of pain which usually comes with the onset of symptoms. It has been attributed to involvement of phylogenetically older pain pathway which receives fibres from both the spinothalamic tract and the trigeminal nerve [6].
The other additional feature in our patient was the presence of contralateral hemiparesis. This variant of Wallenberg’s syndrome was first described by Opalski in 1949. Wallenberg’s syndrome with contralateral hemiplegia is called as Babinski Nageotte syndrome [7]. Some authors question the existence of hemiparesis as they attribute the weakness to represent a spinocerebellar hypotonic syndrome rather than represent a pyramidal tract involvement [1]. But the presence of Babinski sign in our patient suggests pyramidal tract involvement. In fact Hermann et al., have suggested that the eponym Opalski syndrome should be reserved only for those cases with ipsilateral hemiparesis with Babinski positivity and contralateral numbness [8].
The cause of hemiparesis remains controversial. Opalski attributed it to caudal extension of the infarct to involve corticospinal fibres after the decussation. But recent studies suggest that involvement of medullary penetrating arteries which supply the pyramidal fibres post decussation may be responsible. These arteries are a branch of the vertebral artery, which has been identified as being most common artery to be involved in Wallenberg’s syndrome [7].
Another interesting aspect in our patient was the history of alcohol binge preceding the onset of symptom in our patient. There have been studies correlating alcohol with relative polycytosis (PCV of 50.8). The proposed mechanisms include inhibition of ADH release, higher erythropoietin levels in alcoholics and a putative role for nocturnal hypoxia in alcoholics [9].
Alcohol is not an established risk factor for stroke, specifically posterior circulation stroke. But, a study from China has suggested that incidence of posterior circulation stroke is twice as high in alcoholics when compared to general population [10]. More detailed studies are needed before we can establish the role of alcohol in posterior circulation stroke, but it is reasonable to say that it might have played a role in our patient.
Conclusion
LMS can have a diverse clinical presentation and the absence of the classical pattern should not deter one from diagnosing Wallenberg syndrome. Radiology helps in understanding how vascular lesion localisation can lead to changed presentation. Opalski syndrome is a rare variant of Wallenberg’s syndrome with ipsilateral hemiparesis. It should not be diagnosed in the absence of hyperreflexia or Babinski sign. Excessive alcohol intake may play a role in the etiopathogenesis of posterior circulation stroke.
[1]. JS Kim, Pure lateral medullary infarction: clinical - radiological correlation of 130 acute, consecutive patientsBrain. 2003 126(8):1864-72. [Google Scholar]
[2]. RL Sacco, L Freddo, JA Bello, Wallenberg’s lateral medullary syndrome. Clinical-magnetic resonance imaging correlationsArch Neurol 1993 50(6):609-14. [Google Scholar]
[3]. A Vrettos, K Fiotaki, E Galati, A crossed brainstem syndrome without crossed sensory symptomatologyBMJ Case Reports 2013 doi:10.1136/bcr-2012-006709. [Google Scholar]
[4]. P Cerrato, D Imperiale, M Bergui, Restricted dissociated sensory loss in a patient with a lateral medullary syndrome: A clinical-MRI study.Stroke. 2000 31(12):3064-66. [Google Scholar]
[5]. SQ Zhang, MY Liu, B Wan, HM Zheng, Contralateral body half hypalgesia in a patient with lateral medullary infarction: atypical Wallenberg syndromeEur Neurol 2008 59(3-4):211-15. [Google Scholar]
[6]. RD Currier, CL Giles, RN Dejong, Some comments on Wallenberg’s lateral medullary syndromeNeurology. 1961 11:778-91. [Google Scholar]
[7]. S Pandey, A Batla, Opalski’s syndrome: A rare variant of lateral medullary syndrome.J Neurosci Rural Pract 2013 4(1):102-04. [Google Scholar]
[8]. DM Hermann, HH Jung, CL Bassetti, Lateral medullary infarct with alternating and dissociated sensorimotor deficits: Opalski’s syndrome revisitedEur J Neurol 2009 16(9):e72-4. [Google Scholar]
[9]. M Messinezy, TC Pearson, Apparent polycythaemia: diagnosis, pathogenesis and management.Eur J Haematol 1993 51(3):125-31. [Google Scholar]
[10]. SS Yang, JP Jia, Differences in risk factors between anterior and posterior circulation affecting young ischemic stroke onset and prognosis.Zhonghua Yi Xue Za Zhi 2013 93(5):348-51. [Google Scholar]