Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are severe cutaneous adverse reactions (SCAR) which are frequently caused by exposure to drugs and cause significant morbidity and mortality. A careful literature search revealed that only a few reports of diclofenac induced and one case of serratiopeptidase associated case report of SJS or TEN have been published till date. However, to our knowledge, no case report of diclofenac-serratiopeptidase combination induced SJS have been published till date. In this backdrop, we describe the first case of a 62-year-old woman who developed diffuse, erythematous rash on face, trunk and both extremities which later turned into blisters following five day treatment with diclofenac and serratiopeptidase combination. There was extensive ulceration of buccal, genital and ocular mucosa. The body surface area involvement of the patient at the time of presentation was 9%. A provisional diagnosis of SJS was made by the treating physician. After administration of intravenous antibiotic, topical antiseptic, anti-histamine, topical lubricants, fluid therapy and parenteral nutrition patient recovered and were discharged.
Case Report
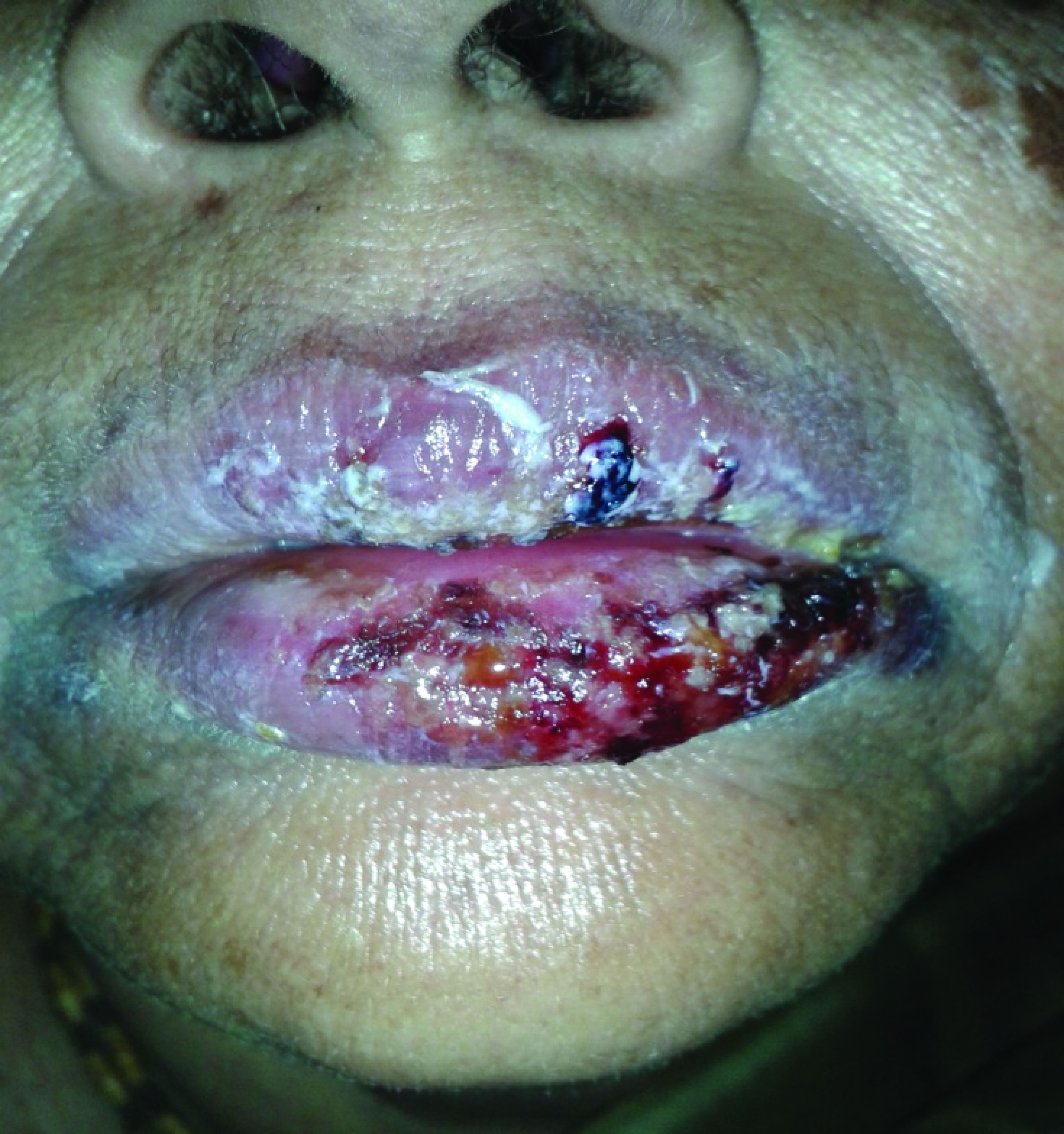
A 62-year-old female patient was admitted in the emergency with blisters and bulla [Table/Fig-1,2] over the body since six days after taking diclofenac and serratiopeptidase combination for relief of pain over heel. She was a known hypertensive since last seven years for which she was on regular anti-hypertensive medications. She had no history of drug allergic responses in the past [Table/Fig-3]. She developed fever, stinging eyes and discomfort upon swallowing after four days drug exposure of diclofenac and serratiopeptidase Sectioncombination twice daily. The next day she noticed erythematous rash over face and trunk. The rash spread and became generalised, with the appearance of bulla or blisters. There was ulceration of the buccal, genital and ocular mucosa (varying grade). She then consulted a primary care physician, who stopped the NSAIDs combination and advised her to get admitted to a tertiary care hospital immediately (day 7). On examination, the patient was alert and appeared dehydrated. Her body temperature was 38.9°C, blood pressure was 110/70 mm Hg, pulse rate was 92 beats per minute, and respiratory rate was 20 breaths per minute.
There were fluid filled blisters and ulcers in oral, nasal and genital cavity. Tenderness was evident, especially in parts of the skin affected by the blisters or erosions. Ocular involvement was gradual from acute conjunctivitis, eyelid oedema, erythema, crusts, and ocular discharge. Nikolsky’s sign was positive. The body surface area involvement of the patient at the time of presentation was 9%.
The patient’s white blood cell count was 5,200/mm³, the haemoglobin concentration was 10.8 g/dL, and the platelet count was 1,75,000/mm³. There was elevated erythrocyte sedimentation rate (ESR) (61 mm/h; reference range 0–15). Her C-reactive protein was increased to 7.1 mg/dL. The level of total protein was 6.0 g/dL, the level of albumin was 3.2 g/dL, the level of blood urea nitrogen was 13 mg/dL, and the level of serum creatinine was 0.5 mg/dL, and elevated random blood glucose 209 mg/dl. Serum total IgE 8.3 (1.5- 378.0 IU/ mL), ECG and X-ray chest were normal. The sodium and potassium levels were slightly decreased. There was no evidence of eosinophilia and levels of liver enzymes and serum bilirubin were within the normal range. Blood culture was negative.
Serological tests for HIV, HBV and HCV were negative. A serological evaluation of patient was also done for HSV and the titers were not significantly elevated thus ruling the possibilities of HSV infection in etiopathogenesis in this particular case.
Histological examination including direct immunofluorescence analysis of the skin biopsy was also done in order to rule out differential diagnoses such as autoimmune blistering diseases like pemphigus vulgaris, bullous fixed drug eruption, erythema multiforme and acute generalised exanthematic pustulosis.
The patient was started antibiotic inj. ciprofloxacin 500mg twice daily, inj. cefotaxime 1gm i.v. to reduce the risk of secondary infection, received fluid therapy and parenteral nutrition with high protein content. Chlorhexidine rinse was given to maintained good oral hygiene and white-soft paraffin was applied on the lips to relieve pain. Oral and nasal crusts were regularly removed, and the mouth was sprayed with antiseptics several times a day. To prevent any ocular sequelae eye drops and physiologic saline were instilled every four hours to avoid development of any synechiae. There was complete recovery of ocular lesions after 10 days.
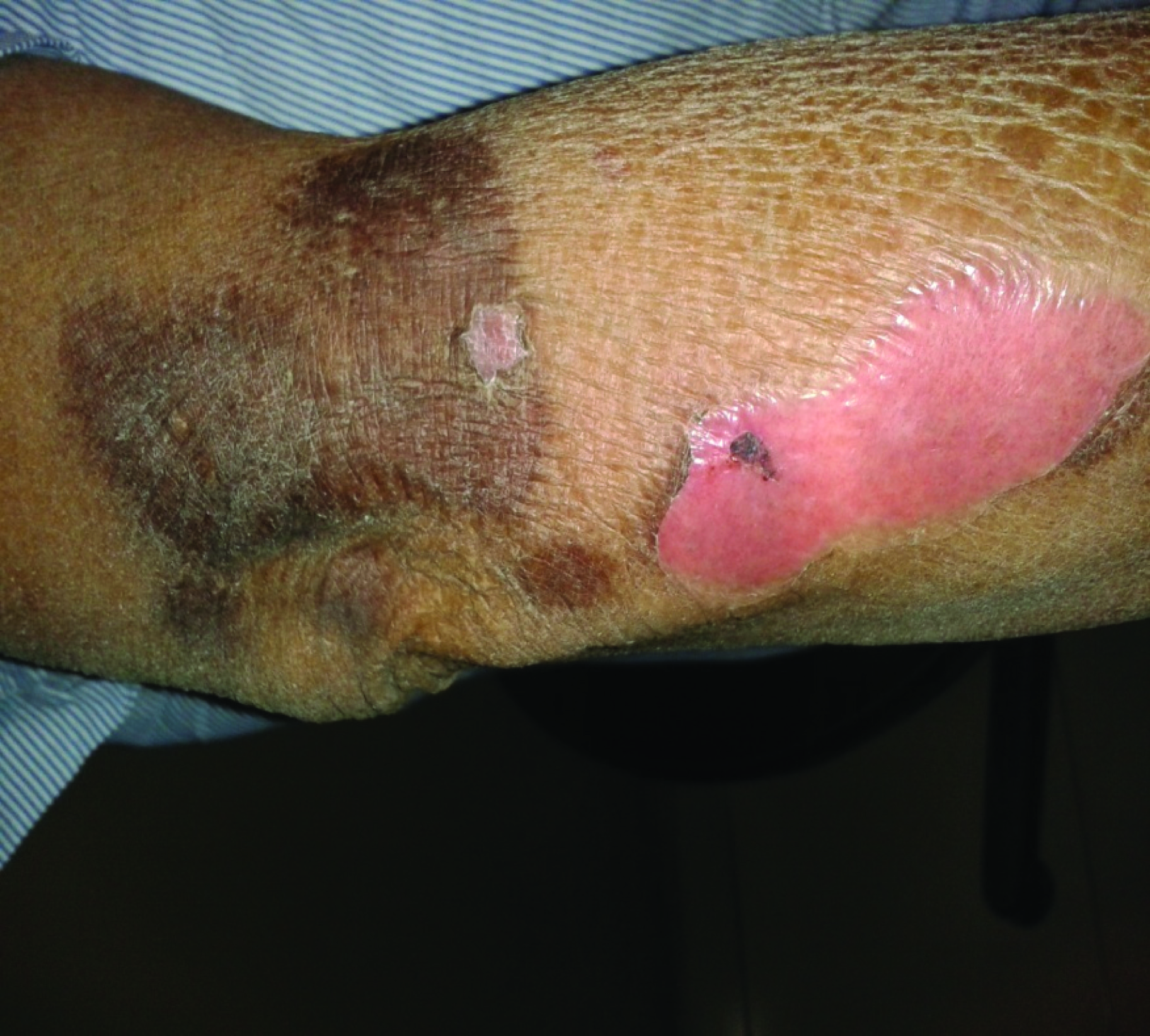
After treatment of skin lesions by the topical application of mupirocin, 0.9% NaCl and 0.5% AgNO3 three times a day for 15 days, the skin condition gradually improved. The patient was discharged from the hospital on day 14. During the hospitalisation, temperature normalized and the skin lesions resolved with mild hypo/ or hyperpigmented spots[Table/Fig-4,5] after three weeks of treatment.
The causality assessment as per the Naranjo algorithm [1] and WHO- UMC criteria [2] revealed the ADR to be Probable (Naranjo score 7). Assessment of causality by using the algorithm of drug causality for epidermal necrolysis (ALDEN) [3] was also used [Table/Fig-6] as this method is specific for SJS. Given that rechallenge tests with medication are contraindicated for SJS, we did not seek to test the effects of rechallenge in our patient.
Shows oedema and crusting of the lips with erythematous purpuric macular lesions
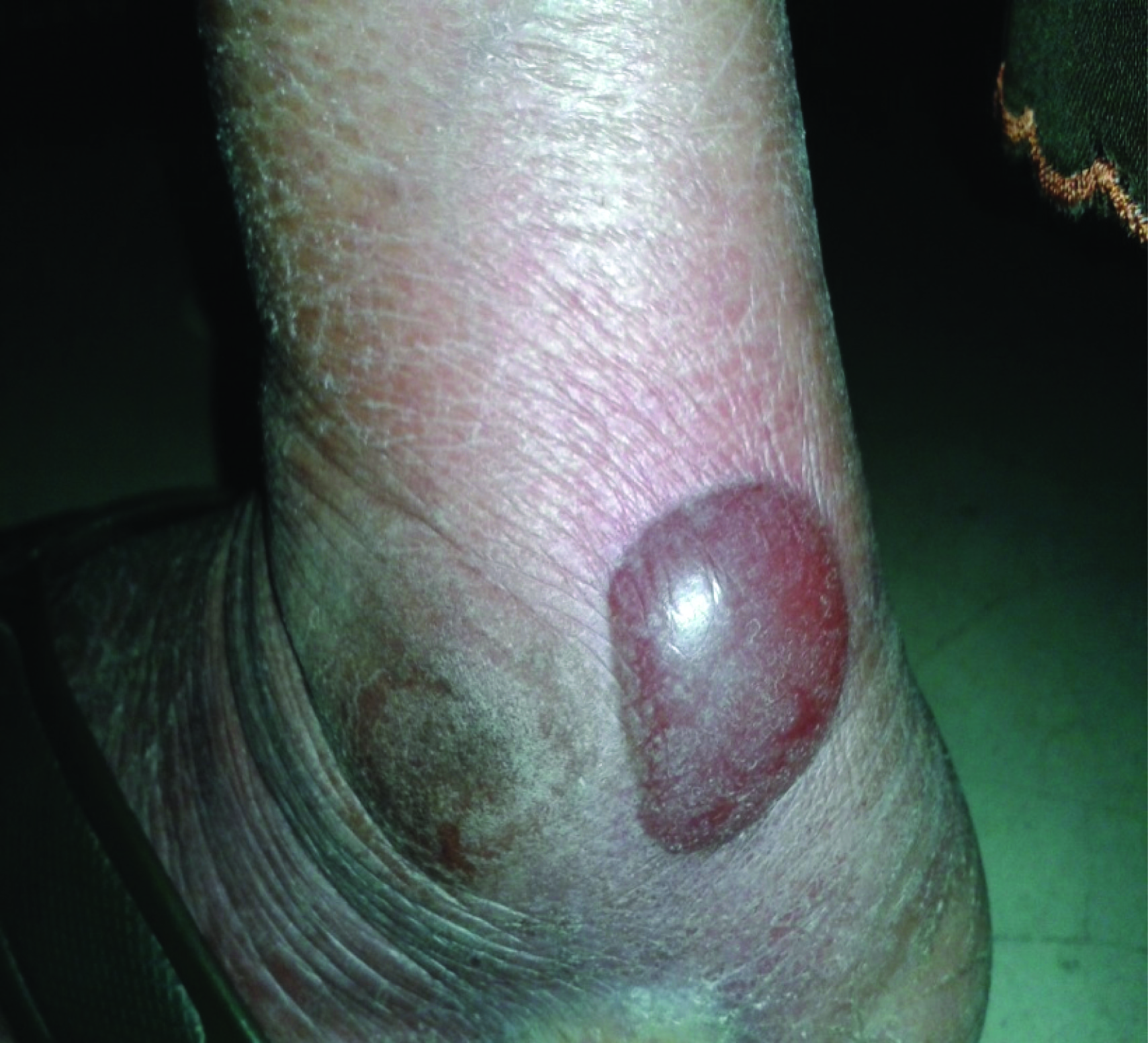
Fluid-filled blisters – flaccid (i.e. not tense)
Report of skin-prick test (drug allergy testing)
| S. No | Drug Name | Reaction |
| 1 | Paracetamol | - |
| 2 | Diclofenac | +++ |
| 3 | Ibuprofen | - |
| 4 | Ciprofloxacin | - |
| 5 | Amoxicillin | - |
| 6 | Metronidazole | ++ |
| 7 | Cephalexin | ++ |
| 8 | Pantoprazole | - |
| 9 | Ranitinide | - |
| 10 | Levofloxacin | ++ |
| 11 | Aspirin | - |
| 12 | Allopurinol | - |
| 13 | Amlodipine besilate | - |
| 14 | Nimesulide | - |
| 15 | Co-trimoxazole | - |
| 16 | Roxithromycin | ++ |
| 17 | Vitamin B complex | ++ |
| [Interpretation: medicines marked with (++/ or +++) are to be strictly avoided; medicines marked with (+) denote marginal sensitivity and are best to be avoided unless deemed absolutely necessary under medical supervision and medicines marked (-) have not produced any localized reaction at the time of testing] |
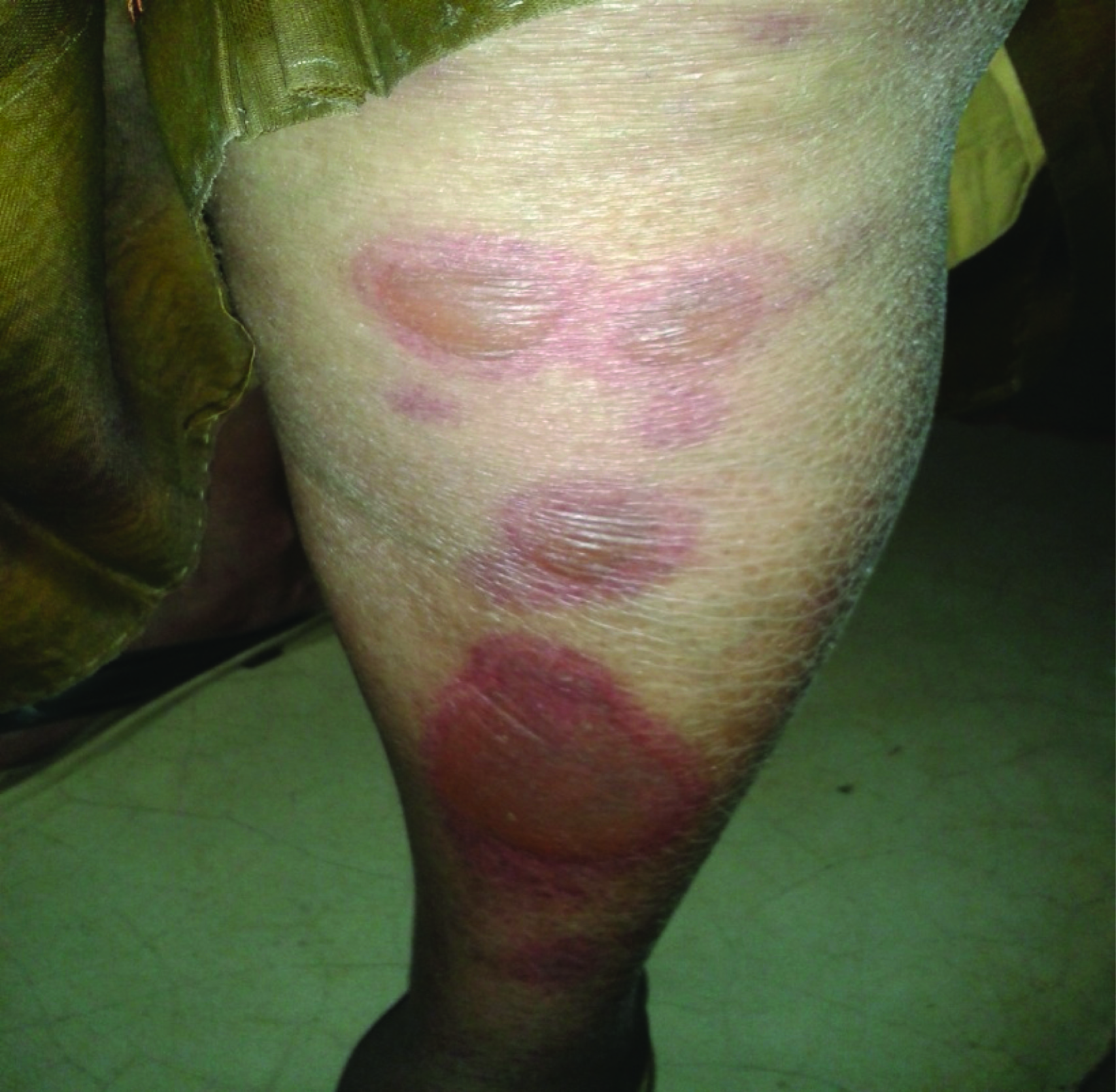
Flat atypical target lesions
Mucocutaneous lesions at forearm during healing
Assessment of causality by using the algorithm of drug causality for epidermal necrolysis (ALDEN)
| Criterion | Values Rules to apply | Score in the present case |
| Delay from initial drug component intake to onset of reaction (index day) | Suggestive +3 From 5 to 28 days↵
Compatible +2 From 29 to 56 days↵
Likely +1 From 1 to 4 days↵
Unlikely −1 >56 Days↵
Excluded −3 Drug started on or after the index day↵
In case of previous reaction to the same drug, only changes for:↵
Suggestive: +3: from 1 to 4 days↵
Likely: +1: from 5 to 56 days | Suggestive +3 |
| Drug present in the body on index day | Definite 0↵
Doubtful −1↵
Excluded −3 | Definite: 0 |
| Prechallenge/rechallenge | Positive specific for disease and drug: 4↵
Positive specific for disease or drug: 2↵
Positive unspecific: 1↵
Not done/unknown: 0↵
Negative −2 | Not done: 0 |
| Dechallenge | Neutral 0 Drug stopped (or unknown)↵
Negative −2 Drug continued without harm | Neutral: 0 |
| Type of drug (notoriety) | Strongly associated 3 Drug of the “high-risk” list according to previous case–control studies↵
Associated 2 Drug with definite but lower risk according to previous case–control studies↵
Suspected 1 Several previous reports, ambiguous epidemiology results (drug “under surveillance”)↵
Unknown 0 All other drugs including newly released ones↵
Not suspected −1 No evidence of association from previous epidemiology study with sufficient number of exposed controls | Associated: 2 |
| Other cause | Possible −1 Rank all drugs from highest to lowest intermediate score↵
If at least one has an intermediate score >3, subtract 1 point
from the score of each of the other drugs taken by the patient (another cause is more likely) | 0 |
(Interpretation: <0, Very unlikely; 0–1, unlikely; 2–3, possible; 4–5, probable; ≥6, very probable)
Total score in present case=5, means “probable”
Severity-of-illness was assessed by using the SCORTEN criteria are
| Prognostic factors | Points | SCORTEN ORTEN (sum of individual scores) | Predicted mortality (%) | Score in present case |
| Age > 40 | Yes = 1, No = 0 | 0-1 | 3.2% | 1 |
| Heart rate >120/min | Yes = 1, No = 0 | 2 | 12.1% | 0 |
| Cancer or haematologic malignancy | Yes = 1, No = 0 | 3 | 35.8% | 0 |
| >10% body surface area | Yes = 1, No = 0 | 4 | 58.3% | 0 |
| Serum urea >10mm/L | Yes = 1, No = 0 | >5 | 90% | 0 |
| Serum bicarbonate<20mm/L | Yes = 1, No = 0 | | | 0 |
| Serum glucose >14mm/L (1 mmol/L of Glucose = 18.02 mg/dL) | Yes = 1, No = 0 | | | 0 Total score= 1 |
Discussion
SJS and TEN, rare variants of severe adverse cutaneous drug reactions are characterized by more or less extensive painful erythematous macules evolving to epidermal detachment and mucous membrane erosions resulting from massive apoptosis of epithelial cells [4]. Previously, they were thought to be discrete entities with different pathogenesis. Now, we know that SJS/TEN may be a continuum depending on the extent of body surface area (BSA) affected by epidermal detachment (>30% in established TEN; 10-30% in SJS/TEN overlap and <10% in SJS), and the extent of mucosal involvement (at least 2 mucosal surfaces comprising ocular, oral and genital). The pathogenesis of SJS/TEN is not fully understood but is believed to be immune-mediated [5].
Diclofenac is a widely used non-steroidal anti-inflammatory drug (NSAID) that exhibits anti-inflammatory, analgesic, and antipyretic activities. Although, hypersensitivity to diclofenac is rare, cutaneous reactions, such as urticaria, angioedema, skin rash or blisters with fever, toxic epidermal necrolysis, erythema multiforme, exfoliative dermatitis, Stevens-Johnson syndrome and anaphylaxis have been described following exposure to the drug [6]. Serratiopeptidase is a potent anti-inflammatory enzyme. Supplementing with these enzymes with NSAIDs has shown to have a favourable anti-inflammatory effect on the tissues of the body [7]. According to a review of the literature, it appears that very few cases of SJS have been reported as being associated exclusively with diclofenac usage [8] and one exclusively with serratiopeptidase [9], but no case of SJS/TEN is reported with this combination.
Diagnosis relies mainly on clinical signs together with the histological analysis of a skin biopsy showing typical full-thickness epidermal necrolysis due to extensive keratinocyte apoptosis. Differential diagnoses include autoimmune bullous dermatoses, acute generalized exanthematous pustulosis, erythema multiforme (EM), disseminated fixed bullous drug eruption and staphyloccocal scalded skin syndrome.
Lesions of EM minor are characterised by single mucosal ulcerations and typical target lesions of skin, was excluded. EM major shows ulcerations involving more than one mucous membrane with skin target lesions. Typical target skin lesions are necessary along with mucosal ulcerations to consider diagnosing them as either EM minor or major. These lesions can be triggered by HSV infections or adverse drug reactions [10]. SJS is a more severe condition characterised by wide spread small blisters on torso and mucosal ulcerations with atypical skin target lesions triggered by drug intake. EM is usually triggered by herpes simplex infections, but rarely by drug intake, whereas SJS is mainly drug-induced. Herpetic ulcers are smaller with regular borders than ulcers associated with SJS. The presence of a temporal relationship between the drug intake and onset of the disease excludes the possibility of any infectious aetiologies. Absence of desquamative gingivitis excluded pemphigus vulgaris. Absence of Wickham’s striae in ulcerated area, excluded bullous lichen planus lesions [11].
Due to the high risk of mortality, management of patients with SJS/TEN requires rapid diagnosis, evaluation of the prognosis using SCORTEN [12] [Table/Fig-7], rapid identification and interruption of the culprit drug, specialized supportive care ideally in an intensive care unit, and the consideration of immunomodulatory agents such as high-dose intravenous immunoglobulin.
Prompt identification and withdrawal of the culprit drug(s), early diagnosis, evaluation of the severity and prognosis of disease, rapid initiating supportive care in an appropriate setting is the mainstay for the management of SJS/ TEN [12]. Awareness of prescribers of high-risk drugs, close monitoring for drug eruptions/ systemic symptoms, and the use of simple tests like the full (complete) blood count, tests of renal and liver function, with immediate cessation of the culprit drug may be the most cost-effective method where specialized tests and specialist care are not readily available. Nonetheless, all health professionals and patients should be educated on potential danger symptoms and signs of SJS/TEN to look out for when taking high-risk drugs. This will go a long way in preventing long-term psychological sequelae in survivors, and disruption of the doctor-patient relationship in clinical decision making.
Conclusion
The sudden onset, positive drug history, stinging eyes, difficulty in swallowing, erythematous rash over face and trunk, fluid filled blisters and ulcers in oral, nasal and genital cavity, cracking and fissuring of lips with bloody crusting and typical target skin lesions lead to the confusion of drug-induced SJS or EM. We have consulted dermatologist to avoid this confusion. Final diagnosis of drug-induced SJS was established based on the positive drug history, clinical appearance, distribution of the lesions and biopsy report.
In summary, this report of diclofenac-serratiopeptidase combination induced SJS underscores the importance that physicians bear in mind that SJS can be developed within only a few days after ingestion of over-the-counter medications, such as diclofenacserratiopeptidase.
(Interpretation: <0, Very unlikely; 0–1, unlikely; 2–3, possible; 4–5, probable; ≥6, very probable)Total score in present case=5, means “probable”
[1]. CA Naranjo, U Busto, EM Sellers, P Sandor, I Ruiz, EA Roberts, A method for estimating the probability of adverse drug reactions.Clin Pharmacol Ther 1981 30:239-45. [Google Scholar]
[2]. The use of the WHO-UMC system for standardised case causality assessment. Available from: http://www.WHO-UMC.org/graphics/4409.pdf (Last accessed on 2013 Dec 30). [Google Scholar]
[3]. B Sassolas, C Haddad, M Mockenhaupt, A Dunant, Y Liss, K Bork, ALDEN, an algorithm for assessment of drug causality in Stevens-Johnson Syndrome and toxic epidermal necrolysis: comparison with case-control analysisClin Pharmacol Ther 2010 88:60-68. [Google Scholar]
[4]. S Bastuji-Garin, B Razny, RS Stern, NH Shear, L Naldi, JC Roujeau, Clinical classification of cases of toxic epidermal necrolysis, Steven-Johnson syndrome and erythema multiforme.Arch Dermatol 1993 129:92-96. [Google Scholar]
[5]. Thong Bernard Yu-Hor, Stevens-Johnson syndrome / toxic epidermal necrolysis: an Asia-Pacific perspectiveAsia Pac Allergy 2013 3:215-23. [Google Scholar]
[6]. Diclofenac potassium. http://www.drugs.com/pro/diclofenac-potassium.html (accessed on 7th Feb’2014). [Google Scholar]
[7]. A Mazzone, M Catalani, M Constanzo, A Drusian, A Mandoli, S Russo, Evaluation of Serratia peptidase in acute or chronic inflammation of otorhinolaryngolog pathology: a multicentre, double-blind, randomized trial versus placeboJ Int Med Res 1990 18(5):379-88. [Google Scholar]
[8]. SR Shetty, L Chatra, P Shenai, PK Rao, Stevens-Johnson Syndrome: a case report.Journal of Oral Science. 2010 52(2):343-46. [Google Scholar]
[9]. H Kanetomo, F Kazuyoshi, H Toshio, A case of Stevens-Johnson Syndrome induced by serratiopeptidase (Dasen).Skin Research 1991 30(6):646-49. [Google Scholar]
[10]. H Assier, S Bastuji-Garin, J Revuz, J Roujeau, Erythema multiforme with mucous membrane involvement and steven-johnson syndrome are clinically different disorders with distinct disordersArch Dermatol 1995 131:539-43. [Google Scholar]
[11]. RM Williams, RC Cocklin, multiformea Erythema, Review and contrast from Stevens- Johnson syndrome/toxic epidermal necrolysisDent Clin North AM 2005 49:67-76. [Google Scholar]
[12]. S Bastuji-Garin, N Fouchard, SCORTEN: a severity-of-illness score for toxic epidermal necrolysisJ Invest Dermatol 2000 115:149-53.:149-53. [Google Scholar]