Cancer of the breast is one of the commonest carcinomas in women and cause of cancer death in western world. In India also, the incidence of breast cancer is fast approaching to that in the western world [1,2] and accounts for the second leading cause of death in women, the first being carcinoma of cervix [1,3]. The high frequency of breast cancer in women has prompted an intensive study of possible modifiable risk factors (clinical parameters, morphological typing, and biological markers) for assessment of prognosis, prevention strategies, and treatment modalities [1].
Treatment of breast cancers is increasingly dependent on the biological phenotype of the tumours. For example, breast cancers expressing the estrogen receptor are highly responsive to Tamoxifen and those over-expressing the erbB2 oncoprotein are responsive to Herceptin. Cells with normal p53 are able to delay progression from the G1 to the S phase of the cycle while abnormal DNA is repaired. The p53 protein is a DNA binding protein localized to the nucleus, which functions primarily by controlling the transcription of several other genes responsible for growth of the cell and also mediates cell cycle arrest and apoptosis [4]. Cells with the inactivated, stabilized, or mutant p53 protein cannot delay and thus the replication of abnormal DNA cannot be prevented [5].
The ability of p53 to control apoptosis in response to DNA damage has important therapeutic implications. The two major modalities of cancer treatment like radiation therapy and chemotherapy, mediate their effects by inducing DNA damage and subsequently apoptosisis triggered in the tumour cells that retain normal p53 genes. By contrast, tumours which frequently carry p53 mutations are relatively resistant to chemotherapy and radiotherapy. Data clearly indicates that p53 overexpression in breast cancers confers poor prognosis and poor response to endocrine therapy and chemotherapy [6]. Loss of p53 function is associated with poor outcome, i.e., ≈50% increased risk of recurrence or death and it is one of the independent, poor prognostic factors in breast cancer.
Normal p53 protein has a very short half-life and thus the protein level is too low to be identified immunohistochemically. In contrast, most mutant p53 proteins have a longer half-life and are easily detected by immunohistochemical methods [5]. Arbitrary cut-off for getting p53-postivity in breast cancers is 10%, but about a third or more have mutated p53 gene [7]. This is because that only certain mutations yield a stable protein whereas other mutations lead to a truncated protein which is not detected by IHC.
Sometimes wild-type normal p53 may accumulate in tumours as a result of a response to other DNA damage or by binding to other cellular proteins, giving a false positive IHC result. The correlation between p53 accumulation measured by IHC and p53 mutation detected by sequencing has been estimated to be less than 75% in breast cancers Norberg etal., [8]. Nearly 13 antibodies to p53 are available and the sensitivity of different antibodies to p53 is variable. The different sensitivities of these antibodies range from 18-36%.
This study was undertaken to study the immunoexpression of p53 protein in 50 available cases of ductal carcinoma of the breast, by immunohistochemistry and correlating the results with clinical and histological parameters. The study also aimed to determine the incidence of malignant neoplasms of the female breast with respect to age, sex and location.
Materials and Methods
One hundred sixty two cases were diagnosed carcinoma of breast on Hematoxylin and Eosin stained sections in the Department of Pathology, Rajah Muthiah Medical College-Annamalai University, during 2006 to 2009. Most of the patients diagnosed as carcinoma of breast were referred to higher centre along with their paraffin blocks for further evaluation and treatment and therefore available 50 cases of tissue materials and their clinical parameters were included in this study, which was carried out for 3 years. The subjects included in this study were women in the age group of 25 years to 75 years which includes postmenopausal and premenopausal women. The study was carried after obtaining the college research committee approval. The histopathology material included trucut biopsies, wedge biopsies, lumpectomy specimens, and radical mastectomy specimens. The patient’s clinical details like age, and location of the tumour were collected from the medical records department (MRD). The data were analysed to find out the histological types of carcinoma of breast, mode of clinical presentation and age incidence. p53 immune-expression in 50 cases of carcinoma of breast was detected using IHC.
Tumour grading
Haematoxylin and eosin-stained sections of the formalin-fixed, paraffin-embedded tumours of the available 50 cases of invasive ductal carcinomas were independently graded by Nottingham combined histological grading system based on the formula (NPI = [Size of the tumour mass (cm) X 0.2 + Lymph node stage (1-3) + grade (1-3)]). In accordance with this grading system, each tumour was assessed and scored numerically for the percentage of tubule formation, the degree of nuclear pleomorphism and the mitotic count in 10-high power fields. The scores obtained for each morphological parameter were added to obtain an overall grade. Tumour size, tumour grade, lymph node status and other prognostic parameters were recorded and tabulated.
Immunohistochemistry
Four-micron sections from 50 cases of paraffin blocks of breast cancers were subjected to immunohistochemical studies using Super Sensitive Non-biotin HRP Detection System using biogenex anti p53 protein [D07] from mouse. When the 50 cases were subjected to p53 IHC, controls were also tested simultaneously. In our primary IHC analysis, positive p53 staining in any percentage of cancer cells was considered to be positive. In a secondary IHC analysis, IHC-positive samples were sub-classified with regard to immunostaining intensity and extent [9] into 3 categories: Dark nuclear staining of more than 10% of tumour cells was scored as p53 overexpression (p53+), weak nuclear staining of (1-10)% of tumour cells was scored as intermediate p53 overexpression (p53), and remaining group showing less than 1 percent stained nuclei was scored as negative. The data recorded was correlated. The results were compared with those of similar studies.
Results
A total of 50 patients, diagnosed with carcinoma breast were included in the final analysis. Age wise distribution of breast cancers is shown in [Table/Fig-1]. [Table/Fig-2] shows the details with regard to tumour side and size. [Table/Fig-3] denotes the percentage of breast cancers with lymph node metastasis. [Table/Fig-4] shows the analysis of various prognostic indicators for carcinoma breast. [Table/Fig-5] shows the percentage of positivity for p53 expression. Association between age groups and outcome parameter is analysed in [Table/Fig-6]. Association between tumour related parameters (side and size) and p 53 status is analysed in [Table/Fig-7]. [Table/Fig-8] analyses the association between lymph node metastasis and p53 status in the 50 cases. The association between various prognostic indices (tumour grading, Nottingham index, lymphocyte reaction and necrosis) and p53 status is analysed in [Table/Fig-9]. The data obtained was analysed by Logistic Regression Method. [Table/Fig-10] shows Grade 1 and Grade 2 infiltrating ductal carcinoma of breast. [Table/Fig-11] shows Grade 3 infiltrating ductal carcinoma of breast. [Table/Fig-12] shows overexpression of p53 immunohistochemistry in infiltrating ductal carcinoma of breast. [Table/Fig-13] shows weak p53 staining in infiltrating ductal carcinoma of breast and [Table/Fig-14] shows negative p53 staining in infiltrating ductal carcinoma of breast.
Percentage age distribution of study participants (n=50)
| Age groups | Frequency | Percent |
|---|
| 35 yrs & below | 7 | 14.0 |
| 36 to 50 yrs | 29 | 58.0 |
| Above 50 yrs | 14 | 28.0 |
Percentage analysis of tumour related parameters (side and size)(n=50)
| Parameter | Frequency | Percent |
|---|
| I. Side |
| Left | 30 | 60.0 |
| Right | 20 | 40.0 |
| II. Size |
| 2cm and below | 10 | 20.0 |
| 2cm to 3 cm | 19 | 38.0 |
| 3cm to 4 cm | 11 | 22.0 |
| above 4cm | 10 | 20.0 |
Percentage analysis of lymphnode metastasis (n=50)
| Parameter | Frequency | Percent |
|---|
| Lymphnode status |
| Negative | 26 | 52.0 |
| Positive | 24 | 48.0 |
Percentage analysis of various prognostic indicators for carcinoma breast like grading, lymphocytic reaction and necrosis (n=50)
| Parameter | Frequency | Percent |
|---|
| Grade |
| Grade1 | 24 | 48.0 |
| Grade2 | 20 | 40.0 |
| Grade3 | 6 | 12.0 |
| NPI |
| less tdan 3.4 | 17 | 34.0 |
| 3.4 - 5.4 | 24 | 48.0 |
| above 5.4 | 9 | 18.0 |
| Lymphocytes reaction |
| Mild | 36 | 72.0 |
| Moderate | 11 | 22.0 |
| Severe | 3 | 6.0 |
| Necrosis |
| Mild | 35 | 70.0 |
| Moderate | 11 | 22.0 |
| Severe | 4 | 8.0 |
Percent age positivity for p53 expression (n=50)
| p 53 | Frequency | Percent |
|---|
| Positive | 11 | 22.0 |
| Negative | 39 | 78.0 |
2Analysis of association between age groups and p53 status (n=50)
| Parameter | p53 | Unadjusted OR | p-value | 95% CI |
|---|
| Positive | Negative | Lower | Higher |
| Age group | 35 yrs and below | 4 57.1% | 3 42.9% | 8.00 | 0.054 | 0.963 | 66.450 |
| 36 to 50 yrs | 5 17.2% | 24 82.8% | 1.25 | 0.806 | 0.211 | 7.414 |
| More than 50 yrs (Base line) | 2 14.3% | 12 85.7% | | | | |
Analysis of association between tumour related parameters (side and size) and p 53 status (n=50)
| Parameter | P53 | Unadjusted OR | p-value | 95% CI |
|---|
| Positive | Negative | Lower | Higher |
| Side | Right (baseline) | 3 15.0% | 17 85.0% | | | | |
| Left | 8 26.7% | 22 73.3% | 2.061 | 0.335 | 0.474 | 8.963 |
| Size | Below 2cm (baseline) | 2 20.0% | 8 80.0% | | | | |
| 2cm to below 3 cm | 4 21.1% | 15 78.9% | 1.067 | 0.947 | 0.159 | 7.145 |
| 3cm to below 4 cm | 2 18.2% | 9 81.8% | 0.889 | 0.916 | 0.101 | 7.856 |
| 4 and above | 3 30.0% | 7 70.0% | 1.714 | 0.608 | 0.219 | 13.406 |
Analysis of association between lymph node metastasis and p53 status (n=50)
| Parameter | P53 | Unadjusted OR | p-value | 95% CI |
|---|
| Positive | Negative | Lower | Higher |
|---|
| Lymph node status | Negative | 6 23.1% | 20 76.9% | 1.140 | 0.848 | 0.298 | 4.365 |
| Positive (baseline) | 5 20.8% | 19 79.2% | | | | |
Analysis of association between various prognostic indices (tumour grading, Nottingham index, lymphocyte reaction and necrosis) and p53 status (n=50)
| Parameter | P53 | Unadjusted OR | p-value | 95% CI |
|---|
| Positive | Negative | Lower | Higher |
|---|
| Grade | Grade1 | 2 8.3% | 22 91.7% | 0.182 | 0.134 | 0.020 | 1.692 |
| Grade2 | 7 35.0% | 13 65.0% | 1.077 | 0.940 | 0.156 | 7.420 |
| Grade3 (Baseline) | 2 33.3% | 4 66.7% | | | | |
| NPI | less tdan 3.4 (Baseline) | 3 17.6% | 14 82.4% | | | | |
| 3.4 - 5.4 | 6 25.0% | 18 75.0% | 1.556 | 0.577 | 0.330 | 7.343 |
| above 5.4 | 2 22.2% | 7 77.8% | 1.333 | 0.779 | 0.179 | 9.912 |
| Lympho-cytes Reaction | Mild (Baseline) | 8 22.2% | 28 77.8% | | | | |
| Moderate | 2 18.2% | 9 81.8% | 0.778 | 0.775 | 0.139 | 4.352 |
| Severe | 1 33.3% | 2 66.7% | 1.750 | 0.664 | 0.140 | 21.876 |
| Necrosis | Mild | 11 31.4% | 24 68.6% | 740426161 .429 | 0.999 | 0.000 | . |
| Moderate | 0 0.0% | 11 100.0% | 1.000 | 1.000 | 0.000 | . |
| 0 0.0% | 4 100.0% | | | | |
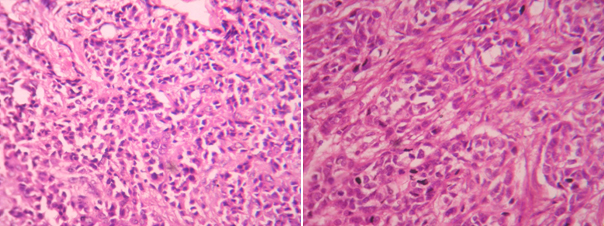
Photomicrograph of invasive ductal carcinoma (H & E, High power view) grade-1 and 2

Photomicrograph of invasive ductal carcinoma (H & E, High power view) grade-3
Photomicrograph of p53 positive ihc slide witd clear overexpression
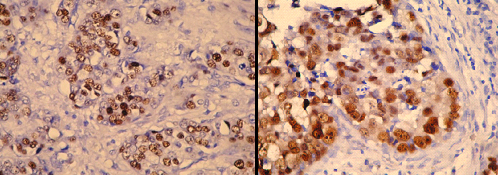
Photomicrograph of p53 positive ihc slide witd weak nuclear staining
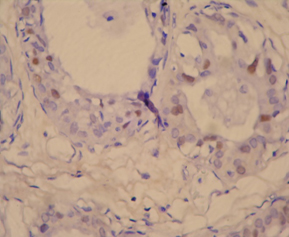
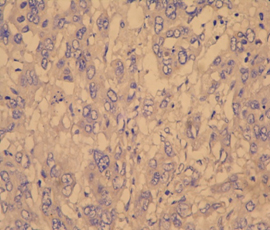
Photomicrograph of p53 positive ihc slide witd negative staining
Discussion
Breast cancer is one of the most frequent cancers in women worldwide and represents over 22% of all malignancies among females [9]. In India, the incidence of breast cancer is increasing and approaching to the western world. Many population based cancer registry data from India has revealed breast cancer as the commonest cancer in woman and has overtaken cervix cancer. This malignancy accounts for 19-34% of all cancer cases among women nationally [10].
In our institute, cervix cancer was found to be the most common malignancy encountered in women (32%) followed by carcinoma of breast (21.37%) among the biopsy materials received.
The age-specific incidence of breast carcinoma overall increases rapidly until the age of 50 years, and then continues to increase at a slower rate for older women, suggesting some key carcinogenic events occur before rather than after menopause [11]. In terms of long term survival the most favoured group in breast cancer patients were in the range of 46-50 years. Above 50 years and below 35 years were a less favourable prognostic group [12]. According to the reports of National Cancer Registry Project (NCRP-ICMR), average age of patients seen in Population Based Cancer Registries (PBCR) were maximum seen in fourth and fifth decades. Carcinoma of breast is rare in women in their 20s or 30s without a family history of breast cancer [13]. The germ line mutations are responsible for two-thirds of familial breast carcinomas or roughly account for 5% of all cases [1].
In this study majority of the participants, 29 (58%) were between 36 to 50 years of age. The proportion of subjects below 35 years and above 50 years was 14% and 28% respectively [Table/Fig-1]. The maximum incidence of breast carcinoma was found in the fourth and fifth decade and they are similar to the studies of Saxena et al., [10] Anderson et al., [11] and reports of National Cancer Registry.
Patients with p53 overexpressing tumours in our present study were younger than those without p53 overexpressing tumours. The odds of p53 positivity were 8.0 times (95% CI 0.96 to 66.45, p value 0.054) in participants aged 35 years and below, when compared with people above 50 years. The similar odds ratio was 1.25 (95% CI 0.2 to 7.4, p value 0.8) times in participants aged 36 to 50 years. [Table/Fig- 6]. This concurs with the study done by Karin et al., and Angela et al., [14,15].
Several studies have documented the peculiar fact that breast carcinoma is slightly more frequent in the left breast than in the right. Similarly, in the present study, 60% were in the left breast and 40% were in the right breast [Table/Fig-2]. There is a study stating that breast cancers present as an ill-defined mass, sometimes adherent to skin or under lying muscle and most common in the left upper outer quadrant [13]. Additionally, in present study the odds of p53 positivity were 2.06 times (95% CI 0.47 to 8.96, p-value 0.33) in people with tumour on the left side, when compared with the right side [Table/Fig-7]. There are no significant studies correlating the p53 oncoprotein positivity with side of the lesion.
In the present study the size of the tumour was 2cm or below in 20% of the subjects. The proportion of subjects with tumour sizes between 2 to 3 cm, 3 to 4 cm and above 4 cm was 38%, 22% and 20% respectively [Table/Fig-2]. The odds of p53 positivity was 1.06 times (95% CI 0.15 to 7.14) in the people with tumour size 2 to 3 cm when compared with the people less than 2 cm tumour size. The odds were 0.89 (95% CI 0.1 to 7.8, p value 0.96) in people with tumour size 3 to 4 cm and was 1.7 (95% CI 0.21 to 13.4, p value 0.60) in people with tumour size more than 4 cm [Table/Fig-7]. Thus it is clear that the increase in the size of the tumour raises the probability of p53 positivity in the breast cancers and thereby p53 becomes significant marker of poor prognosis.
Carcinoma of breast is broadly classified as ductal and lobular neoplasia [16]. Many of the tumours have been referred to as ductal because they do not have the lobular pattern. However, they are not proved to arise in ducts and in practice, they are diagnosed through exclusion because they do not fit into any of the special type categories. For these reasons, the No Special Type (NST) carcinoma is equivalent to the description used by Fisher’s group, Ductal without Special Features or Not Otherwise Specified (NOS) type [17]. In present study, all cases were Invasive Ductal Carcinoma (IDC). The reports by authors Anderson et al., [11], Bland et al., [17], Cutuli et al., [18], Siddiqui et al., [19], Martinazzi et al., [20], shows maximum percentage of cases to be Invasive Ductal Carcinoma - NOS. All the cases studied for p53 immunohistochemistry were Invasive Ductal Carcinoma of No Special Type, and so no correlations were possible between histological type and p53 oncoprotein.
Among the 50 cases, the proportion of subjects with grade 1, 2 and 3 diseases were 48%, 40% and 12% respectively. The proportion of participants with NPI grade less than 3.4, 3.4 to 5.4 and above 5.4 was 34%, 48% and 18% respectively. The lymphocyte reaction was mild, moderate and severe in 72%, 22% and 6% of the study participants. The necrosis was mild in majority (70%) of the cases, moderate in 22% of the cases and severe in the remaining 8% of the cases [Table/Fig-4].The odds of p53 positivity was 0.18 times (95% CI 0.02 to 1.7, p value 0.134) in people with grade 1 and was 1.07 times (95% CI 0.16 to 7.4, p value 0.94) in the people with grade 2, when compared to people with grade 3 disease. The odds were 1.5 times and 1.3 times more in people with NPI between 3.4 to 5.4 and above 5.4 patients respectively than when compared to people with NPI less than 3.4. The odds were 0.78 times (95% CI 0.14 to 4.3, p value 0.78) in tumours with moderate lymphocyte reaction and 1.7 times in tumours with severe lymphocyte reaction (95% CI 0.14 to 21.8, p value 0.66) when compared with people with mild lymphocyte reaction. All the cases of p53 positivity were reported in people with mild necrosis and no cases were reported in moderate and severe necrosis [Table/Fig-9].
p53 was present significantly in carcinomas with intermediate and high-grade or poorly differentiated nuclear grade than in low-grade tumours. These observations about association of mutant p53 protein with high grade cancers (IDC-NOS) do agree with the studies of Martinazzi et al., [20], Roshen et al., [21], and Kilinc et al., [22].
The odds of p53 positivity were 1.1 times (95% CI 0.29 to 4.3, p value 0.84) in people with negative lymph node status, when compared with people with positive lymph node status. This result shows an equivocal p53 status in both the positive and negative cases of lymph node metastases and so no correlations were possible between lymph node metastasis and p53 oncoprotein.
The percentage positivity of p53 immunohistochemistry of the present study is almost similar to the reported statistics of most of the studies [14,20,22–26] except, it is high in studies done by Bhatavdekar et al., [25] and Silvestrini et al., [26]. In maximum number of studies the average percentage of p53 positivity in breast cancers ranges from 18% to 36% [13]. The prevalence of p53 positivity was 22% in this study population [Table/Fig-5].
Conclusion
In this study, the percentage positivity of p53 immunohistochemistry is 22 percent which is almost similar to the reported statistics of various other studies. Patient with p53 overexpressing tumours are seen slightly higher in younger age group less than 35 years, and also in tumours with severe lymphocytic reaction and to those with tumour on the left side. These carcinomas with p53 positivity demonstrated aggressive characteristics, including larger size, higher grade, and necrosis in comparison with p53 negative cases. Data so far clearly indicates that p53 overexpression in breast cancers are mostly aggressive tumours and they confer poor prognosis and likelihood of a poor response to endocrine therapy and chemotherapy [6]. p53 gene mutations of breast cancers are considered to be a good indicator and the strongest predictor of poor outcome of any single factor known presently, including tumour size and proliferation of tumour cells [27].