Reference Intervals denote normative values related to laboratory parameters used by diagnostic centers for clinical diagnosis. The values are obtained by observation or measurement of a particular type of quantity on reference individuals who are selected, as basis for comparison with individuals under clinical investigation, through the use of defined criteria [1].
Prolactin is a protein hormone which is discovered by Oscar Riddle and is encoded by the PRL gene [2]. Human prolactin is secreted from the anterior pituitary gland in both male and female.The release and synthesis of prolactin is episodic and is under neuroendocrinal control mainly through prolactin releasing factor and prolactin inhibiting factor [3]. There are mainly three different forms of prolactin of which the little prolactin (22-kDa) is the biological active form [4,5]. The primary functions of prolactin are to initiate breast feeding and maintain lactation. It also suppresses gonadal function. The determination of prolactin concentration is helpful in diagnosing hypothalamic-pituitary disorders. Micro adenoma (small pituitary tumors) may cause hyperprolactinemia which is sometimes associated with male impotence [6]. Hyperprolactinemia is often associated with galactorrhea and amenorrhea [7]. Prolactin concentration is increased by estrogen, thyrotropin releasing hormone (TRH), renal disease, hypothyroidism, stress, exercise, hypoglycemia and drugs like Chlorpromazine and reserpine. Prolactin level may be lowered by drugs like bromocryptine and L-dopa.
The objective of the present study was to measure the serum prolactin levels in healthy Indian male and female in order to establish reference interval and to compare with the available reference intervals.
Materials and Methods
Reference individuals were selected from those persons who accompanied the patients attending the out patient department of ESIPGIMSR & ESIC Medical College. This cross-sectional study was approved by institutional ethics committee and informed consent was obtained from all the study populations, in accordance with the Declaration of Helsinki. A detailed questionnaire on family history, social status, sexual history, dietary habits, smoking, alcohol intake, history of systemic diseases, and drug history (opiates, chlorpromazine, reserpine, bromocryptine, cimetidine, oral contraceptive pills and L-dopa) were done on all the study subjects. Chronic renal failure, liver disease, pregnancy, lactation, chest wall trauma, primary hypothyroidism, hypoglycemia, pituitary tumor, stress and exercise were ruled out by detailed clinical examination and laboratory investigation to minimize their possible effect on prolactin level. Based on the inclusion and exclusion criteria, a total of 1316 apparently healthy subjects aged between 20 to 79 years including 674 male and 642 female from West Bengal were selected.
Measurement of Prolactin
Two ml of blood were collected by aseptic venepucture technique after 12 hours of fasting and four hours after awakening (pooling equal volume of blood for four times, drawn at six to 18 minute intervals) into a vial containing no anticoagulant. Blood samples were centrifuged after 30 minutes (Time for clot retraction) at 3,000×g for five min. The serum was removed and frozen at -20°C until analysed. Serum prolactin was determined by a microplate immunoenzymometric assay. The reagents were supplied by RFCL Limited. The stated test sensitivity was 0.8ng/ml with negligible cross-reactivity with 1000ng/ml of LH, FSH, TSH, GH and CG.
Prolactins were studied for intra assay and inter assay reproducibility by analysis on pooled control sera. The arithmetic mean (X), standard deviation (SD) and coefficient of variation (CV) were calculated from the individual results of these control series to check the methodical errors. CV of intra assay and inter assay reproducibility were <5.5% and < 4.5% respectively.
Other biochemical tests—fasting plasma glucose, liver function test, thyroid function test, kidney function test (Urea, Creatinine) were also performed.
Statistical analysis was performed using SPSS software. The type of distribution was determined with the Kolmogorov-Smirnov test. Mean, SD, Median and 2.5th and 97.5th percentile with the 0.90. Confidence interval of each percentile values of prolactin were presented along with decade-wise changes.
Results
The reference intervals of serum prolactin in the Indian population was 10.7 ± 3 ng/ml and 11.6 ± 2.8 ng/ml for male and female respectively [Table/Fig-1].
Serum prolactin levels (ng/ml) in reference indian male and female
| Group | n | Mean SD | Median | Min-Max | 2.5th percentile | 97.5th percentile |
|---|
| Male | 674 | 10.7+3 | 10.6 | 2-20.8 | 4.8 | 16.6 |
| Female | 642 | 11.6+2.8 | 11.7 | 1.5-20.9 | 6.1 | 17.1 |
| Total | 1316 | 11.2+2.8 | 11 | 1.5-20.9 | 5.7 | 16.7 |
The 2.5th and 97.5th percentile values of serum prolactin in reference Indian population were 5.7 (0.90 CI = 5.5-5.9) and 16.7 (0.90 CI = 16.5-16.9) respectively [Table/Fig-2].
Percentile values of serum prolactin (ng/ml) levels with 0.90 confidence interval in reference indian male and female
| Group | Percentile | Percentile value | Lower confidence limit | Upper confidence limit |
|---|
| Male | 2.5th | 4.8 | 4.5 | 5.1 |
| 97.5th | 16.6 | 16.3 | 16.9 |
| Female | 2.5th | 6.1 | 5.8 | 6.4 |
| 97.5th | 17.1 | 16.8 | 17.4 |
| Total | 2.5th | 5.7 | 5.5 | 5.9 |
| 97.5th | 16.7 | 16.5 | 16.9 |
Decade-wise analysis of serum prolactin level in reference population showed a steady increase from 20-29 years to advancing decades until 4th decade and thereafter declines steadily [Table/Fig-3].
Decade-wise serum prolactin levels (mean ± sd) in reference indian male and female
| Age group in years | 20-29 | 30-39 | 40-49 | 50-59 | ≥ 60 | Over all |
|---|
| n | Male | 132 | 133 | 141 | 128 | 140 | 674 |
| Female | 130 | 138 | 124 | 124 | 126 | 642 |
| Total | 262 | 271 | 265 | 252 | 266 | 1316 |
| Serum Prolactin (ng/ml) | Male | 10.7 ±2.3 | 10.9 ±2.5 | 10.8 ±3.8 | 10.7 ±2.8 | 10.3 ±3 | 10.7 ±3 |
| Female | 11 ±2.3 | 11.8 ±2.3 | 12.4 ±3.4 | 11.4 ±2.8 | 11 ±3 | 11.6 ±2.8 |
| Total | 10.9 ±2.3 | 11.3 ±2.4 | 12.1 3.3 | 11 ±2.8 | 10.7 ±3 | 11.2 ±2.8 |
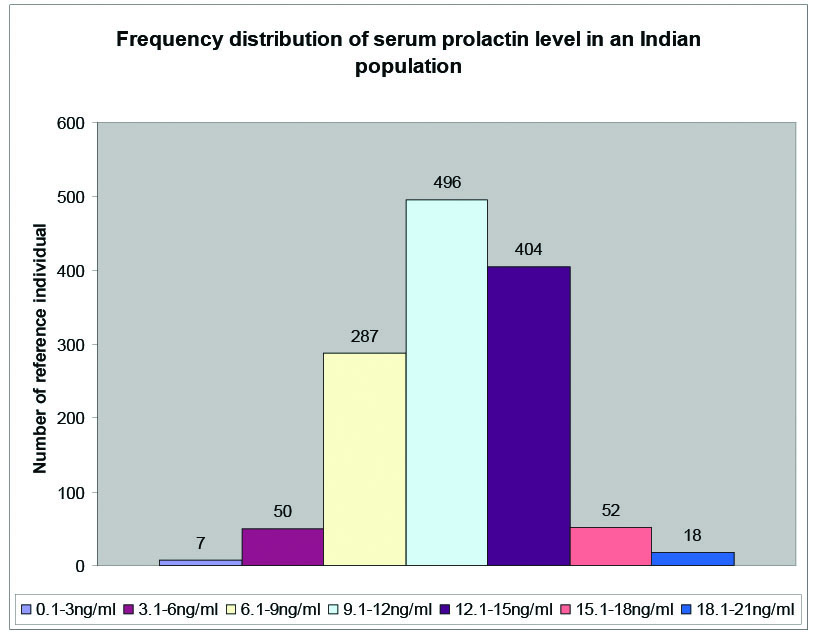
The frequency distribution of serum prolactin levels showed normal Gaussian distribution [Table/Fig-4].
Percentile values of serum prolactin (ng/ml) levels with 0.90 confidence interval in reference indian male and female
Discussion
Reference Intervals denote normative values related to laboratory parameters used by diagnostic centers for clinical diagnosis. International guidelines recommend that every country must establish reference intervals for healthy individuals belonging to a group of homogeneous population. Considering enormous racial and ethnic diversity of Indian population, it is mandatory to establish reference intervals specific to Indian population [1].
Gudrun Wiedemann and L. Jonetz-Mentzel [9] have observed significant difference of prolactin level between the sexes in the age group of 7, 8 and 9 (p = 0.0091, p = 0.0012 and p = 0.0271, respectively) in their study among the sera of 686 healthy neonates, infants, children and adolescents (Age range 5 days to 18 years). They also found the decreased concentration of serum prolactin with increasing age.
M Sachidhanandam et al., [8] have tried to determine the reference range of serum prolactin in only 205 male Rajputs, Gorkhas and South-Indians soldiers with age between 20 to 50 years which did not represent the true in Indian population, moreover they did not follow the recommendation of International Federation of Clinical Chemistry and Laboratory Medicine (IFCC), which states that the establishment of reference intervals requires a minimum of 120 individuals in each subgroup. It is also suggested that for an accurate estimation of reference intervals, the larger numbers are required when partitioning is to be carried out on the basis of age, sex and other factors for establishing reference intervals for any population. Additionally, the reference intervals will also be determined for different age groups separately for male and female gender. Where as this study followed the recommendation of IFCC and established the reference intervals of 1316 apparently healthy subjects aged between 20 to 79 years including 674 male and 642 female from West Bengal [Table/Fig-1] and presented the decade wise changes of serum prolactin with minimum 120 individuals in each sub group [Table/Fig-3].
Abbassi-Ghanavati M et al., have observed that the serum prolactin levels in first, second and third trimester pregnancy were 36-213 ng/ml, 110-130 ng/ml and 137-372 ng/ml respectively due to the high level of circulatory estrogen [10]. Where as this study showed the serum prolactin levels for all ages were 10.7 ± 3 ng/ml in healthy male and 11.6 ± 2.8 ng/ml in healthy female [Table/Fig-1]. The increase level of prolactin in female is mediated by elevated estrogen concentration in female as opposed to male. Decade-wise analysis of serum prolactin level in female reference population showed a steady increase from 20-29 years to advancing decades until 4th decade and thereafter declines steadily due to the difference in the circulating estrogen level before and after menopause although decade-wise analysis of male reference population did not show any such pattern [Table/Fig-3].
Basu M et al., have suggested that prolonged residence at lower as well as at extreme altitude does not appreciably alter blood levels of prolactin in men [11]. Hence forth this reference value is also applicable to a male population residing at high altitude.
Although rare in the general population, macroprolactinemia is frequently found in individuals receiving medical attention [12,13]. Beltran L et al., used the predominant immunoassay platforms for prolactin to assay serum samples treated with polyethylene glycol (PEG) and establish and validate reference intervals for total and monomeric prolactin [14]. But investigations for macroprolactinemia are necessary in sera only when hyperprolactinemia is detected [15].
Limitation
Limitation of our study is that we did not include the paediatric group and pregnant women. Hence, establishment of Reference Intervals for serum prolactin in pediatric group and pregnant women can be done in future.
Conclusion
Reference Intervals for serum prolactin in Indian population will immensely help in diagnosing hypothalamic-pituitary disorders in India. Additionally, the results will be beneficial in formulating our own guidelines for hyper and hypoprolactinemia pertaining to Indian population. Moreover, our study will help each laboratory to formulate their own reference interval for prolactin as of now they were dependent either on the values written on the kit-literature supplied by the reagent manufacturing company or the reference intervals of foreign population.