Background: Early detection of tuberculosis is important for reducing its morbidity and mortality especially in the patients with non-productive cough.
To overcome the cumbersome process involved in collection and processing of the sputum specimen, the time consumed for reporting of sputum by Ziehl Neelsen (ZN) method and to introduce a routine screening test in suspected, symptomless tuberculosis patients, the present study was designed using saliva as diagnostic medium and Auramine Rhodamine (AR) as staining method.
On review of literature, there was no study which has tried diagnosing tuberculosis using saliva with flurochrome stain; hence the present study was designed.
Aim: To introduce a routine screening test for tuberculosis patient using saliva and to determine the diagnostic efficacy of routine ZN staining method and AR fluorescent staining method in sputum and saliva smears of pulmonary tuberculosis patients.
Settings and Design: Laboratory settings and Experimental design.
Materials and Method: Fifty smears samples of sputum and saliva of known cases of pulmonary tuberculosis were stained with routine ZN stain and other with AR fluorescent stain. All the specimens were inoculated into Lowenstein-Jensen culture media. The smears were subjected for scanning of Mycobacterium tuberculous bacilli under X 1000 magnification for ZN stain and X 400 magnification for AR stain by grid pattern proposed by National tuberculosis institute and graded by RNTCP grading system.
Results: All 50 sputum samples showed 100% positivity by ZN and AR stain while only 76% positivity was seen by culture. Of the 50 saliva samples 10% cases were positive by ZN, 76% were positive by AR & 70% by culture method. Statistical analysis using chi square test was done, and the value was found to be statistically highly significant for AR staining technique. (p<0.001)
Conclusion: Saliva can prove to be an important tool for the diagnosis as well as screening of the patients with pulmonary tuberculosis when aided with flurochrome staining method.
Introduction
Tuberculosis is an ancient disease and for many centuries it has been the most important of all human infections in its global prevalence with devastating morbidity and massive mortality. It continues to be a major public health threat worldwide despite the availability of many highly sensitive diagnostic tools and highly efficacious treatment for decades[1]. There were an estimated 8.8 million new cases (incidence) of tuberculosis globally in 2010, 1.1 million deaths among human immunodeficiency virus (HIV)- negative cases of tuberculosis and an additional 0.35 million deaths among people who were HIV-positive. This makes tuberculosis the second leading cause of death among the infectious diseases [2]. A staggering 95% of these cases and deaths occur in the developing countries [3,4]. It is essential to ensure proper and early identification of cases, and good treatment outcomes to be able to limit its transmission and obtain successful tuberculosis control [5]. The diagnosis of pulmonary tuberculosis relies heavily on sputum microscopy and culture method, but the associated discomfort in collection and processing of specimen takes long span to give diagnosis. Moreover culture of Mycobacterium tuberculosis even though the gold standard for diagnosis, involves a minimum period of 6-9 weeks [1]. All these have necessitated the need of simple laboratory technique which provides rapid diagnosis. Studies have shown that smear microscopy is highly specific in settings where tuberculosis is more prevalent [6,7]. Microscopy clearly has many advantages when it comes to speed and feasibility, and if sensitivity could be improved it has the potential to become an even more valuable tool for National tuberculosis Control Programme around the world [8-10]
Recent development in smear microscopy is the advent of fluorescence microscopy [11]. This alternative technique is known to increase the sensitivity (10% higher) when compared with Ziehl Neelsen (ZN) microscopy methods while speeding up the whole process to consume much lesser time [12]. Fluorescent acid fast bacilli (AFB) can be seen at lower magnification and smears can be examined in a fraction (about 25% less) of the time than needed for ZN smears.
Till today of the body fluids only sputum has been widely used as a diagnostics medium in pulmonary tuberculosis. Saliva was never investigated, even though it is more easily available. Though acid fast bacilli microscopy using sputum is simple, inexpensive and provides rapid results, it has some limitations. The threshold for detection of AFB in sputum samples under optimal conditions is between 104 and 105 bacilli per ml. Sensitivity is even more reduced if samples are of poor quality, which is often the case in children and HIV-co infected patients [13,14]. The use of sputum smear as a screening procedure for the diagnosis of pulmonary tuberculosis has recently been criticized following the finding by several large laboratories that up to 55% of specimens with positive smear failed to grow in culture while 30% are smear negative but culture positive [15,16].
Aim
As microscopy is the mainstay of tuberculosis diagnosis in our country, in this study we wanted to compare the sensitivity of ZN staining method and Auramine-Rhodamine (AR) staining method in sputum and saliva smears obtained from pulmonary tuberculosis patients and to introduce a routine screening test using saliva as media and fluorescent microscopy as a tool in the diagnosis of cases of pulmonary tuberculosis so that it can be used as a screening procedure in all the public health centers.
Materials and Methods
A total of 100 sputum specimens were aseptically collected from individual reported in OPD who were suspected to have pulmonary tuberculosis disease on the basis of their presenting symptoms in a span of six months. A suspect was defined as an individual if he/she had complaint of persistent cough for more than three weeks, and /or evening rise in temperature for more than two weeks, and/or body mass index (BMI) less than 16. The collected sputum specimen was transported to the laboratory for detection of M. tuberculosis by ZN stain. Randomly 50 subjects with positive sputum smear microscopy in any grade for M. tuberculosis and showing radiological findings suggestive of pulmonary tuberculosis were selected for the study.
After, thorough clinical examination & consent each selected subject was given two dry, sterile bottles with tight fitting corks for the collection of samples. In one bottle sputum sample of 24 hours duration was collected & stored at 4oC. In another bottle, a single early morning, non-stimulated saliva was collected and subjected to concentration method proposed by Petroff [17].
For the preparation of smear clean, fresh and dry, thin (1mm) glass slides free from scratches were used; as scratches may retain flurochrome and appear as florescent artifacts [18]. A smear was prepared with sputum and sediment of saliva on the center of the slide with the help of wire loop and immediately dry fixed by flaming. Four smears, two each from saliva and sputum were prepared.
One each slide from the saliva and sputum were stained with ZN and AR respectively. Since the accuracy of any diagnostic technique depends on the use of standard quality chemicals, it is very essential that stains and reagents of good quality only are used. For the ZN stain, solutions were prepared and staining was done as per the technique described in Bancroft and AFB demonstrated by oil immersion lens using x1000 magnification were bacilli appeared as red rods against blue background [Table/Fig-1] [6,19]. The organisms were regarded as typical M.tubercle bacilli if they were strongly acid fast, slender rods without excessive beading or barring, averaging 2-6 micron in length.
The fluorescent dye solution was prepared and staining was done as per the technique recommended by Matthaei with the addition of potassium permanganate as counter stain [18]. The slides were air dried and examined on the day of staining under fluorescent microscope (Olympus) at x100 magnification. The bacteria fluoresced as reddish golden yellow rods on dark background [Table/Fig-2]. Artifacts tended to appear hazy yellow or grey green and lacked the reddish tinge and were poorly delineated. Although the organisms tended to appear larger than expected due to fluorescent glow, they retained their slightly curved rod like structure. The characteristic features of M. tubercle bacilli were conformed under oil immersion lens using x1000 magnification.
Evaluation of smears was carried out according to the standardized procedure for scanning by grid pattern proposed by national tuberculosis institute [20]. The stained slides were examined, and were reported negative when no AFB were seen in at least 100 microscopic fields. Smears were graded positive for any of the following observations: when 1 to 9 AFB were seen in 100 microscopic fields (scored as scanty positive), when 10 to 99 AFB were seen in 100 fields (scored as 1+), when 1 to 10 AFB were seen per field in at least 50 fields (scored as 2+), and when more than 10 AFB were seen per field in at least 20 fields (scored as 3+) [11]. Sputum and saliva smears were examined separately by two laboratory technicians without knowing the results of each other. A total of 10% slides from both methods were re-examined by the laboratory supervisor and his classification was final in case of discrepancies.
All the specimens of sputum and saliva inoculated into Lowenstein- Jensen culture media were incubated at 37°C for six to eight
weeks in a vertical position for the better development of individual colonies. When small and buff colored colonies grew on LJ medium, the sample was considered as positive.
Data entry and statistical analysis
All calculations were performed using Microsoft office 2005 version for Windows. The Chi-Square test was used to compare the sensitivity of the different techniques.
The study protocol was approved by the Ethical Review Committee of the institution. All study subjects were enrolled in the study only after they had provided informed written consent.
Saliva smear stained with Ziehl Neelsen stain showing positive M.tuberculous bacilli as red rods.
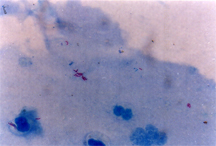
Saliva smear stained with Auramine-Rhodamine flurochrome stain showing positive M.tuberculous bacilli as yellowish glowing rods.

Comparison of grading of saliva and sputum smears
| Grade | Sputum | Saliva |
| | ZN | AR | ZN | AR |
| 3+ | 11 | 16 | 00 | 00 |
| 2+ | 20 | 25 | 00 | 14 |
| 1+ | 18 | 09 | 00 | 09 |
| Scanty | 01 | 00 | 05 | 18 |
| Negative | 00 | 00 | 45 | 09 |
Comparing the positivity rate by zn, ar & lj culture
| | ZN | AR | Culture |
| SPUTUM | 100% | 100% | 88% |
| SALIVA | 10% | 76% | 60% |
Results
All the 50 sputum samples were positive by AR as well as ZN stain as known cases were selected for the study. Among the ZN positive specimens, 1 (2%) was scanty positive, 18 (36%) were 1+, 20 (40%) were 2+ and 11 ( 22%) were 3+, as of the positive AR specimens 9 (18%) , 25 (50%) , 16 (32%) were in grades 1+, 2+ and 3+ respectively [Table/Fig-3].
While of the 50 saliva samples 41 (82%) were positive by AR and only 5 (10%) were positive by ZN stain. Among the ZN positive specimens, all were in scanty positive grade as of the positive AR specimens 18 (36%), 9 (18%), 14 (28%), were in grades scanty, 1+ and 2+ respectively [Table/Fig-3].
Thus, grading of AFB between the slides prepared from same sample using ZN & AR method was compared [Table/Fig-3].
In grade 3+ there were 11 (22%) positive samples and in grade 2+; 20 (40%) positive sample by ZN method ,which increased to 16 (32%) and 25 (50%) in the same grades respectively by AR method, thus indicating the increase in number of bacilli count by later method in sputum smear [Table/Fig-3].
Saliva sample of all the 50 patients, when stained by ZN method showed weaker positivity (10%) , but when stained with AR method, the positivity was much more with almost 82% cases being positive [Table/Fig-3].
Out of 50 sputum samples which were positive by both ZN & AR method, only 44 (88%) sample showed positive growth on LJ culture media while out of 41 cases of saliva samples which were positive by AR method, 38 (76%) cases showed growth on LJ culture media [Table/Fig-4].
Three (6%) saliva specimens which were negative by ZN as well as LJ culture media, were positive by AR staining method only and 9 (18%) saliva sample were negative by all the three method[Table/Fig-3].
Statistical analysis using chi square test for comparison of results of saliva specimen by ZN, AR & LJ culture method was done. The value was found to be statistically highly significant for AR method (p<0.001).
Discussion
Early detection of tuberculosis is important for reducing its morbidity and mortality. Since last few years, flurochrome staining with AR dye has overtook ZN method when large number of specimens has to be scanned as it causes less strain on eyes, is less time consuming and more effective as compared to ZN method [20] .
The use of saliva has been ignored till today, in the diagnosis of tuberculosis, but the present study showed that saliva when aided with FM has simplified the difficulties encountered with sputum smear microscopy like lack of adequate sample in patients with non-productive cough, cumbersome process involved in collection and processing of the samples, collection of specimens over a period of 24 hrs thereby delaying the diagnosis .In the present study it was observed that in case of sputum smears although all the 50 cases (100%) were found to be positive for tubercle bacilli by both ZN & AR staining; scanning of AR stained sputum smears showed increased number of bacilli count per field by the use of fluorescent 44microscope. The similar result was observed by Richards and Associates, Kent PT, Cattamanchi A, also compared the reliability & convenience of fluorescence microscopy with conventional ZN stain and sputum smears [9,10,20,21] .
However, the results of the present study were inconsistent with the findings of R. Kumar, Mithlesh Agrawal & M. Prasad who concluded culture to be more superior over ZN and AR staining method [20,22] . In the present study 12% sputum specimens were negative in culture. From the clinical data it was revealed that these patients were taking anti tuberculosis drugs while collecting specimen. During treatment only the dead / killed bacteria remain in the respiratory specimen (sputum) which were detected by microscopy but were unable to grow in LJ media [1] . The absence of growth was due to non-viability of bacilli. Thus, even though culture is considered to be the reference method by many authors like Heifets LB the assessment based on it has limitations [8] . Moreover this method is very slow, laborious and expensive. The similar results were found by Kent PT who also compared AR staining method with ZN and culture and concluded culture to be less sensitive than AR staining method [9] .
The main aim of the present study was to study the efficacy of saliva samples by AR staining method. Search of the literature since 1951 failed to reveal any study using saliva as a diagnostic media in the diagnosis of cases of pulmonary tuberculosis. The sputum samples had shown 100% positivity with both ZN & AR staining whereas saliva samples showed only 10% positivity with ZN method but when AR stained saliva smears were examined under fluorescent microscope, the positivity of tubercle bacilli increased remarkably to 82% with increase in number of bacilli per field thus increasing the grade.
Other important observation was, 36 saliva samples which were negative by ZN method were found to be positive by fluorescent staining. Similarly eight more cases of saliva smears were positive by fluorescent staining which were negative by both ZN & culture method. That means total 6% saliva smears were positive only because of AR staining method.
Thus, flurochrome stain definitely has advantage over ZN stain probably because of an added advantage of fluorescent microscope which enables an easier detection of mycobacteria as ‘glowing spot’ even if, present in small numbers, thus making it a more sensitive technique.
Despite some evidence that the fluorescent method can be superior to the ZN method, it is not being performed in peripheral tuberculosis laboratories in low-income countries, because of the following concerns; feasibility of centrifugation in settings with irregular power supply; limited human and financial resources; inadequate training capacity; lack of proper biosafety arrangements and potential biohazard posed by centrifugation [1] . In most resource poor countries like ours where tuberculosis is endemic, most of the microscopy centers are using conventional smear microscopy with ZN for the diagnosis of tuberculosis. However, in the light of our study fluorescent microscopy with AR stain, where appropriate facility is available, should be used for screening of the patients to achieve early diagnosis and ensure greater success of the tuberculosis control programmes. It may not be recommended as an ideal alternative to existing conventional sputum microscopy considering the cost implications and other resource constraints involved with its implementation in high tuberculosis burden countries. However, for the district level laboratories where minimum biosafety arrangements can be made by putting in low cost biosafety cabinets and other constraints can be overcome, this saliva smearing technique can be practiced to ensure better case detection of tuberculosis.
Conclusion
All the sputum smears, though positive by both methods i.e. ZN and AR, the number of bacilli per high power field was much more by the later method. Similarly, the saliva samples, when stained by ZN method showed weaker positivity which was much more by AR stain. Few saliva samples which were negative by both ZN as well as by culture were positive by AR stain.
This proves the efficacy of AR fluorescent technique. Even saliva , which was till today neglected by the physicians, can prove to be an important tool for the diagnosis as well as screening of the patients with pulmonary tuberculosis when aided with flurochrome staining method and observed under fluorescent microscope.
Although, AR staining technique using saliva seems to be an adjuvant diagnostic technique for screening of patients of pulmonary tuberculosis, further investigations with more number of study samples of unknown cases will be needed to establish this method beyond doubt. However, their ease of demonstration and high specificity makes AR staining technique using saliva the best available screening procedure in the arsenal of microbiology.
[1]. MD Mafij Uddin, MD Chowdhury R, S Ahmed, MD Rahman T, Khatun Razia, F Leth, Comparison of direct versus concentrated smear microscopy in detection of pulmonary tuberculosisBMC Research Notes 2013 6(291) [Google Scholar]
[2]. World Health Organization:Global Tuberculosis Control:WHO Report 2011. Available from: http://www.who.int/tb/publications/global_report/2011/gtbr11_ full.pdf webcite [Google Scholar]
[3]. K Robert, WHO declares tuberculosis a global emergencyHealth Horiz. 1993 19:251-2. [Google Scholar]
[4]. tuberculosis Control in Prisons-A Manual for Program Managers;WHO/CDS/ tuberculosis/2000281. Available from: http://whqlibdoc.who.int/hq/2000/WHO_ CDS_tuberculosis_2000.281. pdf webcite Accessed on 14 Aug 2013. [Google Scholar]
[5]. RE Huebner, RC Good, JI Tokars, Current practices in mycobacteriology: results of a survey of state public health laboratories.J Clin Microbiol 1993 31:771-5. [Google Scholar]
[6]. H Albert, Economic analysis of the diagnosis of smear-negative pulmonary tuberculosis in South Africa: incorporation of a new rapid test, FASTPlaquetuberculosis, into the diagnostic algorithmInt J Tuberc Lung Dis 2004 8:240-7. [Google Scholar]
[7]. PG Suarez, K Floyd, J Portocarrero, E Alarcon, E Rapiti, G Ramos, Feasibility and cost-effectiveness of standardised second-line drug treatment for chronic tuberculosis patients: a national cohort study in PeruLancet. 2002 359:1980-9. [Google Scholar]
[8]. LB Heifets, RC Good, Current laboratory methods for the diagnosis of tuberculosis. Washington, DC:Tuberculosis: pathogenesis, protection, and control American Society for Microbiology 1994 85(110.) [Google Scholar]
[9]. PT Kent, GP Kubica, Public Health Mycobacteriology: A Guide for the Level III Laboratory. Atlanta, Ga:US Department of Health and Human Services, Centers for Disease Control 1985 159(84) [Google Scholar]
[10]. A Cattamanchi, J Davis, M Pai, L Huang, P Hopewell, K Steingart, Does bleach processing increase the accuracy of sputum smear microscopy for diagnosing pulmonary tuberculosis?J Clin Microbiol 2010 48:2433-39. [Google Scholar]
[11]. VR Aber, BW Allen, DA Mitchison, P Ayuma, EA Edwards, AB Keyes, Quality control in tuberculosis bacteriology, Laboratory studies on isolated positive cultures and the efficiency of direct smear examinationTubercle. 1980 61:123-33. [Google Scholar]
[12]. D Armstrong, LeD-based fluoroscopy and the ParaLens system: illuminating the future of tuberculosis diagnosticsTuberculosis. 2009 7:17-8. [Google Scholar]
[13]. H Getahun, M Harrington, R O’Brien, P Nunn, Diagnosis of smear-negative pulmonary tuberculosis in people with HIV infection or AIDS in resource-constrained settings: informing urgent policy changes.Lancet. 2007 369:2042-9. [Google Scholar]
[14]. EL Corbett, CJ Watt, N Walker, D Maher, BG Williams, MC Raviglione, The growing burden of tuberculosis: global trends and interactions with the HIV epidemic.Arch Intern Med 2003 163:1009-21. [Google Scholar]
[15]. R Uy, C Yu, M Juco, C Adlawan, G Ruiz, M Velmonte, Clorox concentration technique for the demonstration of acid fast bacilli in the sputumJ Microbiol 1988 17:13-8. [Google Scholar]
[16]. Jr Bass J, L Farer, P Hopewell, R Jacobs, Jr Snider D, Diagnostic standards and classification of tuberculosisAm J Respiratory Crit Care Med 1990 142:725-35. [Google Scholar]
[17]. WHO. Global Tuberculosis control; surveillance, planning. WHO report; 2005. Geneva, World Health OrganizTION (who/htm/tb/2005. 349) 2005 [Google Scholar]
[18]. W Kuper S, R May, Detection of acid fast organisms in tissue section by fluorescent microscopy.J of path and bacteria 1960 79:59-62.. [Google Scholar]
[19]. B Swisher, Microorganism. In: John D. Bancroft: Theory and Practice of histopathological techniques 2003 5th EditionMarilyn gamble:329-30. [Google Scholar]
[20]. R Kumar, Vasantakumari Demonstration of acid fast bacilli in sputum smear examination: a view point.Indian journal of tuberculosis 1995 42:135-7. [Google Scholar]
[21]. Diseases IUATaL: Tuberculosis guide for low income countries. Paris, France:International Union against Tuberculosis and Lung Diseases 1996 [Google Scholar]
[22]. H Miorner, G Ganlov, Z Yohannes, Y Adane, Improved sensitivity of direct microscopy for acid-fast bacilli: sedimentation as an alternative to centrifugation for concentration of tubercle bacilli.J Clin Microbiol 1996 34:3206-7. [Google Scholar]