Status epilepticus (SE) is a major medical and neurological emergency. Despite advances in treatment, it is still associated with significant morbidity and mortality. The working group on SE of the epilepsy foundation has defined SE as: “More than 30 minutes of continuous seizure activity or two or more sequential seizures without full recovery of consciousness between seizures” [1]. Lately it is becoming increasingly recognized that seizure duration of more than 10 minutes can lead to brain damage and duration of seizure activity in definition of SE is being decreased [2]. The incidence of childhood convulsive SE (CSE) in developed countries is approximately 20/100,000/year but it varies according to socioeconomic and ethnic characteristics of the population [3]. Age is a main determinant of the epidemiology of SE and even within the pediatric population there are substantial differences between older and younger children in terms of incidence, etiology, and frequency of SE. SE can clinically manifest as convulsive (tonic clonic, clonic, tonic or myoclonic) or non convulsive (absence, simple partial, complex partial) seizures. Duration of SE is a major determinant of response to antiepileptics and final neurological outcome. The reported mortality at hospital discharge in SE is 9–21%. The short-term mortality (all age groups) rates reported from India and other developing countries range between 10.5% and 28% [4]. Convulsive SE results in severe neurological or cognitive sequelae in 11–16% of patients [4]. The objectives of the study were to determine clinical profile, relative incidence of aetiological factors with reference to age and overall mortality and morbidity in patients of SE admitted in pediatric intensive care unit (PICU) of a tertiary care hospital.
Materials and Methods
A prospective study was conducted in the Department of Pediatrics, Patna medical college and hospital, Patna, Bihar, India from April 2008 to March 2009. Informed verbal consent was obtained prior to their enrolment in the study. A predesigned and pretested performa was used to record the details and a total of 70 patients (42 males and 28 females) were included in the study. Inclusion criteria for the study were [1]:
Patients having continuous seizure activity of more than 30 minutes
Patients having two or more sequential seizures without full recovery of consciousness between seizures.
All the patients between six months and 12 years with SE admitted to pediatric intensive care during the study period were included. Exclusion criteria included patients with seizure duration less than 30 minutes, patients with age <six month and >12 years and those patients in whom seizure duration could not be documented with or without loss of consciousness. Lower limit of six month were selected as patients less than six month were not managed in PICU because of difficulty in maintaining temperature. Patients more than 12 years were not included because most of the patients above that age group were admitted in intensive care unit of the medicine department of the hospital.
The duration of SE was ascertained from a reliable patients’ relative or attendant, medical records, and the referring physician’s note. The time to travel from the place of patients’ home place to the city was also taken into consideration while determining the duration of SE. Seizures were classified as per International League Against Epilepsy Group Definitions [5]. Patient’s demographic details, clinical features, investigations and treatment were noted in a master sheet. The duration of SE before starting treatment was also noted. Aetiology was determined by history, investigations and neuroimaging findings. All the patients were managed in PICU with standard treatment protocols. Patients were given mechanical ventilator support whenever necessary. Continuous EEG monitoring and therapeutic drug monitoring was not done in any of the patients.
The patients of SE were divided according to their aetiology into five groups for analysing the study [6]:
Idiopathic group: Seizures occurring in the absence of acute precipitating central nervous system (CNS) insult or systemic metabolic dysfunction or both.
Remote symptomatic: Seizures occurring without acute provocation in a patient with a prior history of CNS insult known to be associated with increased risk of convulsions e.g. stroke, head trauma, meningitis, presence of static encephalopathy.
Febrile group: In this form of SE there is fever as a sole provocation of SE (fever ≥38.40C).
Acute symptomatic group: Seizure occurring during an acute illness, in which there is a known neurologic insult or systemic metabolic dysfunction. The acute CNS insult could be caused by meningitis, encephalitis, head trauma, cerebral malaria, hypoxic encephalopathy or electrolyte imbalance.
Progressive encephalopathy: Seizures occurring at any time during progressive neurologic diseases. In this group neurodegenerative disease, malignancies not in remission, neurocutaneous syndromes were included.
Outcome was assessed as overall mortality and functional outcome. Functional outcome was assessed by Glasgow Outcome Scale (GOS) at three months. Briefly Score 1 is dead, Score 2 is persistent vegetative stage and patient exhibits no obvious cortical function, Score 3 is severe disability and patient depends on others for daily support, Score 4 is moderate disability and patient is independent as far as daily life is concerned, and Score 5 is good recovery and resumption of normal activities. Outcome was considered poor when GOS was 1–4 while Score 5 was a good outcome [7].
Age was considered as a continuous variable and categorical variables included sex, aetiology and response to treatment. Independent t-test was used for continuous variables and chi-square test for categorical variables. The variables studied for statistical significance included age, sex, duration of SE, aetiological risk factors and mortality.
Results
During the study period 70 patients of SE between six months to 12 years were admitted in the PICU. The mean age was 5.94 years (SD=3.152) [Table/Fig-1]. These patients were categorised into five groups according to their aetiology.
Salient features of patients with SE (n=70)
| Age at presentation | 6 month to 12 years |
|---|
| Mean age ± SD | 5.94 ± 3.15 |
| < 4 years | 30 patients |
| Males /females | 42/28 |
| Seizure type | Generalized tonic clonic | 64 |
| Partial | 6 |
| Morbidity | 8 |
| Mortality | 22 |
Forty two patients (60%) were males and 28 patients (40%) were females. Therefore male to female ratio were found to be 1.5:1 and it was found to be statistically significant (p< 0.001).
Fifty two patients (74.2%) presented with SE without any prior history of seizures and 18 patients (25.7%) had one or more seizure episodes in the past. Therefore, it was observed that association of prior seizure episodes with occurrence of SE was not significant (p>0.005).
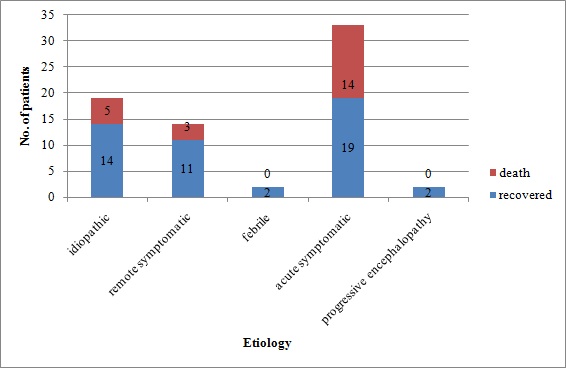
Acute symptomatic group was found to be the most common aetiology for SE and it comprised of 47.14% (33 cases) of total patients. Idiopathic group accounted for 27.14% (19 cases) while remote symptomatic accounted for 20% (14 cases). Both febrile and progressive encephalopathy group had two patients (2.86%). It was also observed that most of the patients of acute symptomatic group were five years or younger.
Patients with age group four years or younger were found to be most vulnerable to develop SE. Thirty patients (42.85%) belonged to this group, out of which 20 patients were from acute symptomatic group (66.6%). Thirteen patients (18.5%) were from four to six years age group and 27 patients were between six to 12 years. In 6-10 years age group idiopathic aetiology was found to be commonest. Out of 14 patients in this age group 7 (50%) were found to be idiopathic. However in 10-12 years age group again acute symptomatic aetiology was more common. Out of 13 patients in this group 6 (46.1%) belonged to acute symptomatic group. Therefore, it was concluded acute symptomatic causes were responsible for most of the cases in less than four years age group and 10-12 years age group i.e. two extremes of the study population.
Maximum patients observed were of generalised convulsive SE i.e. 91.4% (64 cases) while patients of partial SE were 8.6% (6 cases). It was observed that in generalised convulsive SE group, 60.9% (39 cases) were male children while 39.1% (25 cases) were female children (p<0.05). The partial seizure SE was found equally in both sexes i.e. 50% in both sexes.
Irrespective of aetiological cause of SE, the seizure lasted for one to three hours in 37 patients i.e. 52.86% of cases. In 12 patients seizure duration was three to six hours and in 6 patients seizures lasted for more than 24 hours. Remaining patients had either continuous seizure activity or multiple seizure episodes without regaining consciousness between six to 24 hours.
Fever was the most common symptom associated with patients of SE and it was seen in 57 patients (67.14%). In most of the patients it was of high grade (>1010F). Other symptoms were vomiting, unconsciousness, faecal and urinary incontinence. Headache was the commonest symptom when patient regained consciousness and it was complained by 36 patients (51.42%).
As maximum no. of patients of SE were from acute symptomatic group, patients from this group were further categorised according to their respective aetiologies [Table/Fig-2]. It was found that CNS infection was the leading cause of SE especially in younger age group. Viral encephalitis was the commonest aetiology in acute symptomatic group and it constituted 33.33% of cases. It was followed by pyogenic meningitis and tubercular meningitis which contributed 21.21% and 15.15% respectively. Rest of the cases were contributed from cerebral malaria (12.12%), hepatic encephalopathy (9.09%), uremic encephalopathy (3.03%) and dyselectrolytemia (6.07%).
Aetiology of SE in acute symptomatic group
| Aetiology | No. of patients (n=33) | Percentage (%) |
|---|
| Viral encephalitis | 11 | 33.33 |
| Pyogenic meningitis | 7 | 21.21 |
| Tubercular meningitis | 5 | 15.15 |
| Cerebral malaria | 4 | 12.12 |
| Hepatic encephalopathy | 3 | 9.09 |
| Uremic encephalopathy | 1 | 3.03 |
| Dyselectrolytemia | 2 | 6.07 |
The first line drug used in patients of SE for control of seizure was I.V. Lorazepam in all cases. Those in whom seizure recurred even after I.V. Lorazepam, other drugs were used. Intravenous Phenytoin was needed in 27 (38.5%) patients as second line drug. Intravenous Phenobarbitone was used in 18 (25.7%) patients of SE not responding to phenytoin. Nine patients required intravenous midazolam infusion and intravenous levitiracetam. It was observed that in acute symptomatic group SE was better controlled by Phenytoin + Phenobarbitone combination than Phenytoin or Phenobarbitone alone.
Twenty-two patients died with overall mortality rate of 31.4% [Table/Fig-3]. Fourteen deaths were in acute symptomatic group (63.6%), five (22.7%) in idiopathic group and three (1.36%) in remote symptomatic group. There were no deaths in febrile and progressive encephalopathy group. Mortality in acute symptomatic group when compared to other groups was found to be statistically significant (p <0.01).
Mortality in status epileticus
Eight patients (11.42%) emerged with significant neurological deficits. Five Patients developed postencephalitic sequelae with delayed motor milestones and severe mental retardation. Two patients of tubercular meningitis developed focal neurological deficit and speech delay. One patient with pre-existing developmental delay had further deterioration in all the milestones. Patients with poor outcome (GOS 1-4) had significantly longer duration of SE when compared to patients with good outcome (GOS 5) (P< 0.05). Acute symptomatic group had the worst outcome. Out of 33 patients of acute symptomatic group only 16 (48.4%) had GOS five outcome.
Discussion
Most of the data on SE from developing countries was retrospective [8–11]. There are only few prospective studies on SE in pediatric age group [12]. Access to specialist care is a major limiting factor in developing countries because of poor health infrastructure, connectivity, and delays in transportation. A long latency between the onset of status and initiation of appropriate treatment was noted in the series reported from developing countries [9].
In various studies it has been found that males are more vulnerable to develop SE as compared to females. Murthy et al., in their study found that male to female ratio was 1.3:1 [13]. In our study it was found to be 1.5:1. However, no definite causal relationship was found in literature for this male preponderance.
Predominant involvement of younger age group has been reported previously in many studies [14,15]. In our study 43% patients were of 2-4 years age group. Gulati et al., in their study found that 56% of patients were five years or younger [16]. The reason for this predominance of SE in younger children is not known. Probably, mechanisms for control of seizure activity are fragile in younger children and may get disrupted with minimal abnormalities in neurofunction.
The aetiological spectrum of SE in developing countries is distinctly different when compared to developed countries. In this study, acute symptomatic aetiology accounted for 47% of the aetiology. Similar high frequency of acute symptomatic aetiology was reported in the hospital-based series in developing countries [8,9,11,13]. Of the acute symptomatic aetiology, cerebrovascular disease is the predominant cause in developed countries [17,18], whereas in developing countries CNS infections accounted for 28–67% of aetiological spectrum [9,12] and this was much more so in the paediatric age group [8,9]. In the studies from developed countries, the reported frequency of CNS infections as the risk factor varied from 4% to 19% [14]. In our study viral encephalitis was leading cause of SE especially in 2-6 years age group. This was most probably due to increased incidence of Japanese encephalitis in our part of country. It was followed by other CNS infection including meningitis and cerebral malaria. In older children idiopathic aetiology and history of previous epilepsy was more commonly associated.
Experimental studies have shown that the shortest duration of SE that is likely to produce neuronal damage is only 30 minutes whereas the critical duration of SE for the production of irreversible long term clinical sequelae in humans remains unknown [19]. Purpose of aggressive treatment of SE is to shorten the duration of the condition and thus to minimize the neuronal damage caused by noxious systemic and electrical features of SE. In our study most of the seizures lasted between one to three hours and they responded to standard anticonvulsants. SE lasting more than 3 hours and not responding to conventional anticonvulsants were associated with poor final outcome.
Intravenous lorazepam and phenytoin were common antiepileptic drugs used for control of SE in our study. Combination of phenytoin and Phenobarbitone was found to be most effective in controlling SE. The most important adverse effect of combination therapy is sedation and respiratory depression. Because of this life threatening adverse reaction of drugs used in control of SE it is always recommended to manage these patients in PICU setting. The primary aim is to control and abort SE as the duration of seizure activity is directly proportional to immediate mortality and later morbidity. Updated Cochrane review provides some evidence to support the use of intravenous lorazepam in the management of acute tonic clonic convulsions in childhood. It is as effective as and safer than intravenous diazepam in treating acute tonic-clonic convulsions and SE in children [20]. It also provides evidence to support the use of buccal midazolam as the first line treatment of an acute tonic-clonic convulsion and convulsive SE in childhood where intravenous access is not available [20]. This is of particular importance in countries with a high incidence of CNS infectious diseases, where children often present late and in shock (making it difficult to obtain rapid intravenous access) and where intravenous cannula and equipment are in limited supply.
Mortality rate in our study was found to be significantly higher (31.4%) when compared to other studies. In most of the studies mortality rate ranged between 10.5 to 28% [4,21]. However few studies showed mortality rate can be as high as 30% [22]. There are several possible explanations for this higher mortality rate in our study. As our hospital was one of the largest referral centres in the state, several cases were referred from other hospitals and therefore many patients were of refractory SE. Poor literacy rate and lack of transportation led to considerable time lapse between onset of SE and initiation of treatment. As many patients of Japanese encephalitis presented with SE, this contributed to higher mortality rate because of the deadly nature of the disease per se and not due to SE. Younger age group, longer duration of SE and poor response to initial anticonvulsants were associated with higher mortality and morbidity. Our finding of worse outcome in younger patients is consistent with two previous studies which reported higher morbidity and mortality in patients less than three years with SE [6]. In our study with acute cause of SE, there was trend toward worse outcome. Duration of SE is the only potentially modifiable determinant of mortality [23]. Unfortunately, issues that can add to critical minutes, even hours to the timing of initiation and effectiveness of treatment in developing countries are delays in transportation. In our study as well as in the study from Senegal [9] the duration of SE before institution of appropriate treatment was much longer.
Conclusion
The combined effects of SE, disease complications and adverse effect associated with treatment generate a complex situation for the patient. If the duration of SE can be shortened by early treatment, systemic and neurological complications of prolonged seizures may be prevented. Thus, there is an urgent need to train community physicians and the paramedics in the use of intravenous lorazepam and nasal/buccal midazolam in developing countries. The severity and number of co-morbidities also determines prognosis. Mortality rate in SE can vary significantly depending on study population and the settings in which the study is carried out. Infective pathology is still the commonest cause of SE in developing countries. This signifies poor health education and lack of health care facilities in remote areas. There is a need to strengthen the disease specific immunization programme especially in endemic zones for encephalitis. Considering the large number of referrals, upgradation of intensive care facilities even in tertiary care hospital is much sought to improve the overall outcome of SE.