Acute Myeloid Leukemia Presenting as “Bowel Upset”: A Case Report
Smeeta Gajendra1, Ajay Gogia2, Prasenjit Das3, Ritu Gupta4, Pranay Tanwar5
1Attending Consultant, Department of Pathology and Laboratory Medicine, Medanta Hospital, Haryana, India.
2Assistant Professor, Department of Medical Oncology, Dr BRA-IRCH, AIIMS,New Delhi, India.
3Assistant Professor, Department of Pathology, AIIMS,New Delhi, India.
4Additional Professor, Laboratory Oncology Unit, Dr BRA-IRCH, AIIMS,New Delhi, India.
5Assistant Professor, Laboratory Oncology Unit, Dr BRA-IRCH,, AIIMS,New Delhi, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Pranay Tanwar, Room No 425, 4th floor, IRCH, AIIMS, New Delhi-110029, India.
Phone: 9968722016,
E-mail: pranaytanwar@gmail.com, pranaytanwar@aiims.ac.in
Myeloid sarcomas (MS) are the extramedullary presentation of acute myeloid leukemias. At times, they are difficult to diagnose due to lack of any supportive findings in peripheral blood/bone marrow aspirate examination. The involvement of gastrointestinal tract (GIT) by myeloid sarcoma is rare phenomenon. This diagnostic challenge becomes more complex when it is added by vague clinical symptoms. Many times, they have been misdiagnosed as Non-Hodgkin’s lymphoma, small round cell tumour or carcinoma. Here, we are reporting a case of myeloid sarcoma with no haematological abnormality which presented with the symptoms of bowel obstruction and a rare combination of inv. (16) and trisomy 22. The journey to reach the conclusive diagnosis in this case is interesting and sensitizes us to have high index of suspicion in a case, where there is paucity of clinical evidences.
Acute myeloid leukemia, Blast, Intussusception, Myeloid sarcomas, Lymphadenopathy
Case Report
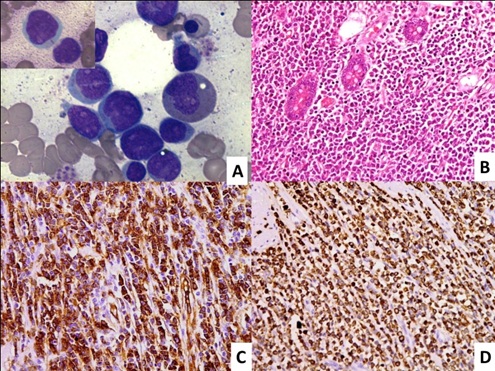
A 35-year-old male presented with repeated episodes of abdominal pain, distension, vomiting and constipation for 3 months. The patient was afebrile with abdominal tenderness and distension without any lymphadenopathy or organomegaly. The patient was evaluated outside of our institution for intestinal obstruction. Ultrasonography showed bilateral pleural effusion with gross ascites and ileo-ileal intussusception. Laboratory examinations including complete blood count, liver and renal function test were within normal limit. On laparotomy, there was adhesion between small bowel loops due to hard intraluminal constricting mass 3x3 cm which was 15cm away from duodenojejunal flexure. Another mass of 5x5 cm was located around 7.5cm proximal to ileocaecal junction and the third ill-defined mass was at ileocaecal junction involving appendix, caecum, terminal ileum and ascending colon. Excision of proximal jejunal mass with jejuno-jejunal anastomosis and ileostomy was done. Excisional biopsy of mass showed monotonous medium sized tumour cell population, resembling lymphoid cell morphology, which were positive for CD45, CD3 and negative for CD19,CD10,CD20,CD5 and CD30, suggesting a diagnosis of T-cell lymphoma. No further action was taken and patient wanted to consult in our institute. After one month patient was presented to our hospital with fatigue and weakness. On examination, he had pallor without any lymphadenopathy or organomegaly. CT scan of abdomen showed circumferential jejunal wall thickening with conglomerate mesentric nodal mass and retroperitoneal adenopathy. The haemoglobin was 90g/L, total leucocyte count was 5.6x109/L and platelet count was 102x109/L. PBS and BMA showed 40% and 80% myeloperoxidase (MPO) positive blasts with many showing auer rods, respectively [Table/Fig-1a]. On flowcytometric immunophenotyping, blasts were positive for CD45, CD34, HLA-DR, cMPO, CD117, CD13, CD33 and CD64; and negative for cCD79a, CD3, CD19 and CD14. The excisional biopsy of small intestine was reviewed in our institution which revealed small intestinal mucosa infiltrated by atypical cells[Table/Fig-1b] with moderate eosinophilic cytoplasm which were destroying the mucosal glands and infiltrating into periserosal fat. On immunohistochemistry (IHC), these atypical cells were immunopositive for CD34 and MPO [Table/Fig-c,d] and negative for CD19, CD20, CD79a, CD3, CD4, CD8, CD56, CD138 and CD30. CG showed inv (16) (p13q22) and trisomy 22. A final diagnosis of acute myeloid leukemia with inv (16) and trisomy 22, preceded by granulocytic sarcoma of small jejunum was made. The patient was started on 3+7 induction chemotherapy and post induction the bone marrow was in morphological remission.
Photomicrograph shows bone marrow aspirate with blast and auer rod (insat) [(1A), Jenner and Giemsa, x100)]. Section from small intestinal wall with diffuse transmural infiltration by a population of discretely lying tumor cells which are infiltrating the mucosal glands. A few eosinophils are also seen [(1B), H&E x100]. These tumor cells are strongly positive or CD34 stain [(1C), IHC (CD34) x100] and Myeloperoxidase stain [(1D), IHC (MPO) x100]
Cases of myeloid sarcoma with AML subtype and associated cytogenetics Authors FAB classification cytogenetics
| Authors | FAB Classification | Cytogenetics |
| Le Beau et al., [4], 1983 | AML-M4 | inv(16) |
| Russell et al., [5], 1988 | AML-M4 | 50,XY,+6,+9,+14,inv(16),+22 |
| Morel et al., [6], 2002 | AML-M2 | inv(16), trisomy 9 and 22 |
| Alvarez et al., [7], 2011 | AML-M2 | CBFβ/MYH11, inv(16) |
| Present case | AML-M2 | inv(16) , trisomy 22 |
Discussion
Granulocytic sarcoma/myeloid sarcoma (MS) is an uncommon tumour composed of myeloid blasts and/or immature myeloid cells at an extramedullary site. MS may occur as an isolated leukemic tumour or precede the appearance of blood or bone marrow disease or may present concurrently with /at relapse of acute myeloid leukemia (AML). It can occur at any anatomical site, most common sites being the skin (13%–22%), bone/spine (9%–25%), and lymph nodes (15%–25%) [1] . Involvement of gastrointestinal tract is rare with the commonest site being ileum (10%-11%) followed by stomach and large intestine [2] . In cases of involvement of gastrintestinal tract (GIT), diagnosis is often delayed due to wide spectrum of vague signs and symptoms such as intermittent abdominal pain, diarrhoea, fever, nausea, vomiting and weight loss [3] . These patients may present with GIT bleeding, perforation or obstruction. The histopathological diagnosis of MS can be challenging, especially when there is no evidence of any neoplastic haematological disorder. It is frequently mistaken for Non-Hodgkin lymphoma (NHL), small round cell tumour or undifferentiated carcinoma. The diagnosis can be facilitated by multiple modalities such as immunoperoxidase stains, immunohistochemistry, conventional cytogenetics (CG), and flow cytometry [2] . Few cases of AML having inv. (16) and other cytogenetic anomalies presenting with MS of GIT have been reported in medical literature previously [Table/Fig-2] [4-7] . It is evident with the literature search that myeloid sarcoma is not so common phenomenon. The combination of cytogenetic anomaly is still rarer. Though, the implication of cytogenetic anomaly on the presenting future have not been correlated yet, still some of them have been complied in [Table/Fig-2]. In the absence of abnormal haematological parameters, histopathological diagnosis of MS is challenging and 47%–56% of patients may be diagnosed as other conditions, most commonly NHL [2]. One of the case [8], of MS in nasopharynx, which was reported as NHL was reviewed after 8months, when patient had persistent thrombocytopenia. Here in this case too, an initial diagnosis of T-cell NHL was made due to rare clinical presentation as intussusception and normal haematological parameter. The CG of this case, revealed inv. (16) along with trisomy 22. This combination of cytogenetic anomaly is rare and has never been reported in medical literature till date. Only one case [9], of MS of breast having trisomy 22 alone has been cited. The treatment options of myeloid sarcoma of gastrointestinal involvement remain the same as that of AML that is, systemic chemotherapy with bone marrow transplantation in addition to surgical resection in appropriate clinical settings. One of the study [10], has concluded, that MS is associated with superior event-free survival and overall survival compared with AML based on the evaluation done on same anti-AML therapy.
Conclusion
MS involving small intestine presenting as intussusception is rare, the histopathological diagnosis may be challenging initially, especially in the setting of normal haematological parameters. Hence high index of clinical suspicion is required to reach the conclusive opinion, so as to minimize the time lag between diagnosis and management.
[1]. M Breccia, F Mandelli, MC Petti, M D’Andrea, E Pescarmona, SA Pileri, Clinico-pathological characteristics of myeloid sarcoma at diagnosis and during follow-up: report of 12 cases from a single institutionLeuk Res 2004 28(11):1165-69. [Google Scholar]
[2]. Kohl SK, Aoun P, Granulocytic sarcoma of the small intestineArchives of Pathology and Laboratory Medicine. 2006 130(10):1570-74. [Google Scholar]
[3]. EK Choi, HK Ha, SH Park, SH Lee, SE Jung, KW Kim, Granulocytic sarcoma of bowel: CT findings.Radiology. 2007 243(3):752-59. [Google Scholar]
[4]. MM Le Beau, RA Larson, MA Bitter, JW Vardiman, HM Golomb, JD Rowley, Association of an inversion of chromosome 16 with abnormal marrow eosinophils in acute myelomonocytic leukemiaN Engl J Med 1983 309:630-36. [Google Scholar]
[5]. SL Russell, FJ Giles, DS Thompson, DJ Scanlon, H Walker, JDM Richards, Granulocytic sarcoma of the small intestine preceding acute myelomonocytic leukemia with abnormal eosinophils and inv (16).Cancer Genet Cytogenet 1988 35:231-35. [Google Scholar]
[6]. F Morel, A Herry, MJ Le Bris, G Le Calvez, V Marion, C Berthou, Isolated granulocytic sarcoma followed by acute myelogenous leukemia type FAB-M2 associated with inversion 16 and trisomies 9 and 22Leukemia. 2002 16(12):2458-59. [Google Scholar]
[7]. P Alvarez, CA Navascués, C Ordieres, M Pipa, IF Vega, P Granero, Granulocytic sarcoma of the small bowel, greater omentum and peritoneum associated with a CBFβ/MYH11 fusion and inv(16) (p13q22): a case reportInt Arch Med. 2011 4(1):3 [Google Scholar]
[8]. J Raphael, A Valent, C Hanna, N Auger, O Casiraghi, V Ribrag, Myeloid sarcoma of the nasopharynx mimicking an aggressive lymphoma.Head Neck Pathol. 2014 8(2):234-38. [Google Scholar]
[9]. F Turpin, M Trassard, A Bourguignat, JL Floiras, V Le Doussal, P Eydoux, An unusual way of manifesting acute granulocytic leukemia: granulocytic sarcoma of the breast. Apropos of a case with trisomy 22.Nouv Rev Fr Hematol 1986 28(2):91-96. [Google Scholar]
[10]. AM Tsimberidou, HM Kantarjian, S Wen, MJ Keating, S O’Brien, M Brandt, Myeloid sarcoma is associated with superior event-free survival and overall survival compared with acute myeloid leukemiaCancer. 2008 113(6):1370-78. [Google Scholar]