Background: Neoplasia results from various genetic and epigenetic factors. Our study focused on the pathogenesis which involved an imbalance in various molecular mechanisms which regulated cell proliferation and apoptosis. The anti-apoptotic mechanism is regulated by Bcl-2 gene, while Ki-67 is expressed exclusively in nuclei of proliferating cells. This study was done to evaluate the basic pathologic process which underlay well and poorly differentiated oral squamous cell carcinoma (OSCC).
Materials and Methods: Thirty cases of oscc were selected, out of which 11 were well differentiated, 9 were moderately differentiated and 10 were poorly differentiated. Three slides of 4μm thickness were prepared out of each sample, which were then subjected to Hematoxylin and eosin stain (H&E) staining and two types of immunohistochemical (IHC) staining. Immunohistochmical markers used were Ki-67 (proliferative marker using MIB-1 (Molecular Immunology Borstel 1) antibody) and Bcl-2 (anti-apoptotic marker). The number of MIB-1 and Bcl-2 positive cells was calculated from ten different high power fields, by counting the number of positive cells per 50 cells in each field, by making a grid pattern. The overall percentage value for each case was evaluated for MIB-1 and Bcl-2 positive cells. Karl-Pearson’s co-relation coefficient was calculated between MIB-1 and Bcl-2 in each group. The aim of this study was to co-relate the expression of Ki-67, a proliferative marker, by using MIB-1 antibody and Bcl-2, an anti-apoptotic marker in various grades of oscc and also to determine whether there was any co-relation between these two markers in the 30 cases of oscc .
Results: A statistically significant increase for MIB-1 and a statistically significant decrease for Bcl-2 was found in well to moderately to poorly differentiated Squamous cell carcinoma (SCC). A statistically significant co-relation was also found between MIB-1 and Bcl-2 in poorly differentiated oscc .
Conclusion: MIB-1 expression is predominant in well, moderate and poorly differentiated SCCs. Bcl-2 expression is predominant in well differentiated than in moderately and poorly differentiated oscc , which suggested that apoptosis probably played a major role in the early stages of carcinogenesis.
Introduction
Squamous cell carcinoma (SCC) of the oral cavity represents the sixth most common cancer worldwide and it accounts for 90% of head and neck lesions [1]. The basis of the carcinoma formation lies in the fact that there is activation of growth promoting oncogenes, inactivation of tumour suppressor genes and alterations in the genes that regulate apoptosis, leading to unregulated cell proliferation and decreased apoptosis. The ultimate progressive growth of tumours and the rate at which they grow, are determined by an excess cell production over cell loss. In tumours with large growth fractions, this imbalance is large, resulting in a more rapid growth than that which is seen in those in which cell production exceeds cell loss by only a small margin. Some tumours result from a high cell turn over, thus implying higher rates of cell proliferation, for example, fast growing tumours like leukaemias and lymphomas, whereas in other tumours, growth occurs due to mutation in the genes which regulate apoptosis, resulting in tumour progression, which is caused by reduced cell death rather than explosive cell proliferation. Such tumours result from a defect in the regulation of programmed cell death (apoptosis), which may contribute to the pathogenesis and progression of cancer [2].
B-cell lymphoma/leukaemia-2 (Bcl-2) gene was first discovered in follicular non-Hodgkin’s B-cell lymphoma. It includes anti-apoptotic Sectionand pro-apoptotic proteins. The anti-apoptotic proteins are Bcl-2 and Bcl-XL, while the pro-apoptotic proteins comprise Bax and Bak. Thus, it is the relative concentration of pro and anti-apoptotic members which decides the actual outcome of a cell which is challenged by an apoptotic stimulus. Over expression of Bcl-2 results in an alteration of programmed cell death, with persistence of cells that fail to die. In normal proliferating epithelium, Bcl-2 is expressed in stem cell zones such as the basal layers, where it acts to prevent the death of cells in the regenerative compartment. Bcl-2 protein overexpression has been found in the early phase of epithelial carcinogenesis [3].
MIB-1 is a monoclonal antibody which was developed by Cattoretti et al., for the detection of Ki-67 antigen, because the original Ki-67 antibody showed immunopositivity only for fresh and frozen tissue sections. MIB-1 recognizes Ki-67 antigen, which is expressed exclusively in nuclei of proliferating cells. (i.e, G1, S, G2 and M phases) [4].
Though, both cell proliferation and a decreased rate of apoptosis are involved in pathogenesis of carcinogenesis, in this study, an attempt was made to determine the predominant mechanism of carcinogenesis in varying grades of oscc , by evaluating the IHC expressions of Bcl-2 and Ki-67 with the use of MIB-1 antibody.
Materials and Methods
The study sample consisted of 30 cases of histologically verified scc of the oral cavity. Out of them, 11 cases were of well-differentiated, 9 were of moderately differentiated and 10 were of poorly differentiated OSCC. The sections were obtained from the archives of Rajiv Gandhi Cancer Hospital, Delhi, India. In each case, three sections, each of 4μm thickness, were taken. One section was stained with H&E stain [5] for histopathological verification and confirmation of the histopathological grade of scc . Of the other two sections which were taken on a poly-L-lysine coated slides, one was subjected to immunohistochemical (IHC) staining for MIB-1 expression and the other was subjected to IHC for Bcl-2 expression [6]. IHC was done by using Biocare Medical Company antibody kit and Biocare MACH 1 Universal HRP-Polymer Kit for both the markers [Table/Fig-1,2and3].
For counterstaining, slides were dipped in Gill’s Haematoxylin for 2-3 minutes, cleared in acetone and xylene and finally mounted with DPX (Distyrene 80 Dibutyl Pathylate Xylene). Its expansion was minimal. During imunohistochemical procedure, normal oral mucosa was used as a negative control, while reactive lymph nodes were used as positive controls.
The slides were subjected to IHC staining and were studied under 4X, 10X and 40X by using motic BA 400 software. The numbers of MIB-1 and Bcl-2 positive cells were noted in 10 different high power fields, by counting the number of positive cells per 50 cells in each field. The 50 cells from each field were counted by making a grid pattern (by the help of motic software), which divided the field into 10 equal divisions and from each division, five good cells with proper cell outlines were chosen, in order to reduce bias. The above-mentioned data was recorded on a master chart for analysis. Percentage value for each case was calculated for MIB-1 and Bcl-2 positive cells. Means and standard deviations for the markers, MIB-1 and Bcl-2 in each group of well, moderate and poorly differentiated SCCs were calculated and Karl-Pearson’s correlation coefficient was calculated between MIB-1 and Bcl-2 in each group [Table/Fig-4,5,6and7].
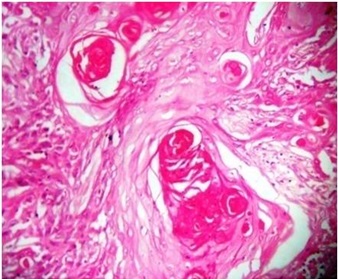
H & E staining in OSCC using H & E staining X40
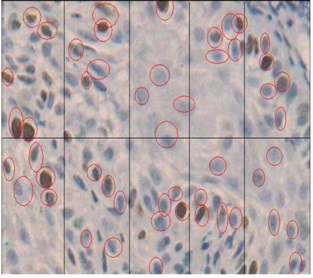
Histological staining in well differentiated SCC using MIB-1 marker X40
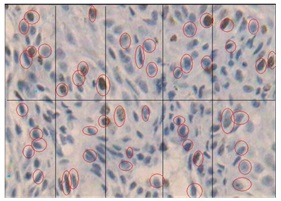
Histological staining in well differentiated SCC using Bcl-2 marker X40
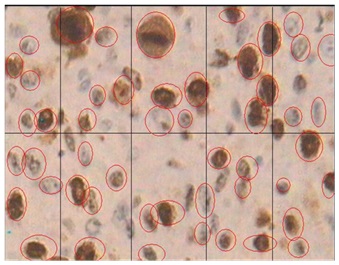
Histological staining in moderately differentiated SCC using MIB-1 marker X40
Histological staining in moderately differentiated SCC using Bcl-2 marker X40
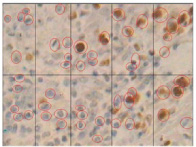
Histological staining in poorly differentiated SCC using MIB-1 marker X40
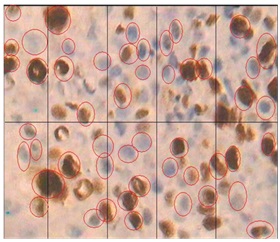
Histological staining in moderately differentiated SCC using Bcl-2 marker X40
Table showing the percentage values of MIB-1 and Bcl-2 expression in 11 cases of well differentiated, 9 of moderately differentiated and 10 of poorly differentiated OSCC
| Cases | Well-Differentiated OSCC | Moderately Differentiated OSCC | Poorly Differentiated OSCC |
| MIB-1 | Bcl-2 | MIB-1 | Bcl-2 | MIB-1 | Bcl-2 |
| 1 | 51.8% | 8.4% | 72.8% | 6.6% | 86.4% | 3% |
| 2 | 61.6% | 8.6% | 68.4% | 6.8% | 70% | 4.2% |
| 3 | 67.6% | 11.8% | 79% | 7.6% | 78.2% | 2.8% |
| 4 | 53.2% | 11.8% | 77.4% | 5.8% | 81.8% | 2.6% |
| 5 | 71.2% | 7.4% | 71% | 5.6% | 78.8% | 4.6% |
| 6 | 56.4% | 6.5% | 72.2% | 6.2% | 87.4% | 4.6% |
| 7 | 54% | 7.6% | 68% | 5.6% | 71.6% | 3.8% |
| 8 | 67.4% | 13.6% | 73.6% | 6.8% | 76.8% | 2.2% |
| 9 | 68.2% | 7.2% | 77.6% | 4.8% | 88.6% | 3.2% |
| 10 | 51.8% | 9.8% | - | - | 76.4% | 4.6% |
| 11 | 58.6% | 10.2% | - | - | - | - |
Line diagram showing the mean values of MIB–1 and Bcl–2 marker in well, moderate and poorly differentiated OSCC
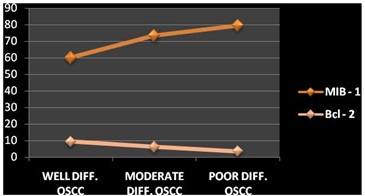
Table showing the mean values ± standard deviation and the co-relation co-efficient of MIB-1 and Bcl-2 expression in well, moderate and poorly differentiated OSCC
| Group | Mean ± SD for MIB-1 | Mean ± SD for Bcl-2 | Co-relation between MIB-1 and Bcl-2 | p-value | |
| Well Differentiated SCC (n=11) | 60.1636 ±7.3424 | 9.3545 ±2.2818 | -0.0979 | p>0.05 | Insignificant |
| Moderately differentiated SCC (n=9) | 73.3333 ±3.9799 | 6.2022 ±0.8426 | 0.0716 | p>0.05 | Insignificant |
| Poorly differentiated SCC (n=10) | 79.5848 ±6.4377 | 3.5623 ±0.9131 | -0.1597 | p <0.05* | Significant |
Results and Observation
It was observed that there was a statistically significant increase for MIB-1 immunostaining and a statistically significant decrease for Bcl-2 immunostaining in well to moderately to poorly differentiated SCCs [Table/Fig-8]. A strong negative co-relation between MIB-1 and Bcl-2 expressions was found in poorly differentiated SCCs. A weakly negative co-relation was found between MIB-1 and Bcl-2 expressions in well differentiated SCCs and a weakly positive co-relation was also observed in moderately differentiated SCCs [Table/Fig-9]. A statistically insignificant co-relation was found in well and moderately differentiated SCCs, while a statistically significant co-relation was found in poorly differentiated SCCs [Table/Fig-10].
Discussion
The expressions of MIB-1 and Bcl-2 were evaluated in various grades of histologically diagnosed oscc cases. In our study, MIB–1 antibody was used in order to detect Ki–67 expression, as Ki-67 had a major drawback that it could be used only for fresh and frozen sections. Ki-67 human nuclear antigen is expressed during the G1, S, G2 and M phases of the cell cycle, but it is not expressed in the quiescent G0 phase [7].
The expression of proliferating marker, MIB-1, showed a statistically significant increase in well to moderately to poorly differentiated SCCs, which was in accordance with the study done by Zidar [8], who suggested that the proliferative fraction progressively increased with the grade of carcinoma and tumour results, due to abnormal proliferation and maturation of epithelial cells. Studies done by Xie et al.,[9] and Guimaraes et al., [10] also showed a co-relation between high values of Ki-67 expression, with a poor prognosis in SCCs of tongue.
The expression of anti-apoptotic marker, Bcl-2, showed a statistically significant decrease in well to moderate to poorly differentiated OSCCs, which was in accordance with the studies done by Loro et al., [11] and Schoelch et al., [12], where dysregulation of Bcl-2 and loss of its expression were noted with increasing grades of carcinoma. The downregulation of Bcl-2 expression, with increasing grades of SCC’s, indicated that this oncoprotein could play a role in relatively early events which were seen in the development and progression of oscc [13].
However, in contrast to these findings, study done by Solomon et al., [14] showed an inverse relationship between Bcl-2 and levels of differentiation of OSCCs. Authors justified these findings, as there was over-expression of Bcl-2 and loss of function of p53, which played important roles in tumourigenesis of oral cancers, as they resulted in defective apoptosis and in subsequent progression of tumour.
A statistically significant co-relation was found between MIB-1 and Bcl-2 expression in poorly differentiated OSCCs. A similar finding was observed by Piattelli et al., [6], where a significant co-relation was found between MIB-1 and Bcl-2. According to this study, proliferative activity was highest, while anti-apoptosis was lowest in case of invasive carcinomas.
Staibano et al., [15] found a low positivity for the proliferating marker, combined with a high positivity for Bcl-2 protein, which correlated with better clinical outcomes in OSCCs. This study was conducted in order to get a better understanding of the dominant, basic, pathologic process which underlay the development of well differentiated oscc , as opposed to that existent in poorly differentiated lesions. An attempt was made to correlate the histological grade of the tumour with the pathogenesis.
Alterations in the levels of proteins pertaining to cell proliferation and apoptosis, are strong indicators of malignant potential in OSCCs and they help in visualizing early stages of oral carcinogenesis [16]. Abnormal Bcl-2 protein favours cell accumulation in tumour cells by allowing them to escape apoptosis, whereas increased Ki-67 protein promotes abnormal proliferation [17,18]. Detection of proliferative index and apoptotic abnormalities with the use of such markers, even before the consequences become clinically or histologically evident, will greatly enhance the potential for making an early diagnosis [19]. Studies which have larger sample sizes should be done, which will help in understanding both the markers better and also aid in deciding the treatment plan.
Conclusion
Although proliferation is predominant in well, moderately and poorly differentiated lesions, anti-apoptosis is the dominant mechanism in the well differentiated than in moderately and poorly differentiated lesions. Further investigation is needed to collaborate the expression of various oncogenes during development and progression of oral neoplasia, so as to open avenues for therapeutic modalities.
[1]. CJ Vicente, JML Junqueira Gutierrez, HA Zapatero, FFM Forcelledo, HG Vallejo, LSJ Arranz, Prognostic significance of p53 expression in oral squamous cell carcinoma without neck node metastasisHead Neck 2004 26:22-30. [Google Scholar]
[2]. V Kumar, KA Abbas, N Fausto, J Aster, Robbins Cotran Pathologic Basis of Disease. 2005 7th EditionWB Saunders:270-340. [Google Scholar]
[3]. LL Loro, GK Vintermyr, AC Johannessen, Cell death regulation in oral squamous cell carcinoma: methodological considerations and clinical significanceJ Oral Pathol Med. 2003 32:125-38. [Google Scholar]
[4]. G Cattoretti, MH Becker, G Key, Schlliter Duchrow, Monoclonal antibodies against recombinant parts of Ki-67 antigen (MIB-1 and MIB-3) detect proliferating cells in microwave processed formalin fixed paraffin sectionsJ Pathol. 1992 168:357-63. [Google Scholar]
[5]. DJ Bancroft, M Gamble, Theories and Practice of Histological Techniques 2002 5th EditionChurchill living stone:130-31. [Google Scholar]
[6]. A Piattelli, G Rubini, M Fioroni, Prevalence of p53, bcl-2 and Ki-67 immunoreactivity and apoptosis in normal oral epithelium and in premalignant and malignant lesions of the oral cavityJ Oral Maxillofac Surg 2002 60:532-40. [Google Scholar]
[7]. Gerdes. Ki-67 and other proliferation markers useful for immunohistochemical diagnostic and prognostic evaluation in human malignanciesSemin Cancer Journal 1990 1:199-206. [Google Scholar]
[8]. N Zidar, N Gale, A Cor, V Kambic, Expression of Ki-67 antigen and proliferative cell nuclear antigen in benign and malignant epithelial lesions of the larynx.Journal of Laryngology and Otology 1996 110:440-45. [Google Scholar]
[9]. X Xie, Petter FPO Clausen, DP Angelis, M Boysen, The prognostic value of spontaneous apoptosis, Bax, Bcl-2, and p53 in oral squamous cell carcinoma of the tongueCancer. 1999 86(6):913-20. [Google Scholar]
[10]. GC Guimaraes, ML Leal, RS Campos, C Zequi Sde, FP Fonseca, IW Cunha, Do proliferating cell nuclear antigen and MIB-1/Ki-67 have prognostic value in penile squamous cell carcinoma?Urology. 2007 70(1):137-42. [Google Scholar]
[11]. LL Loro, OK Vintermyr, Oral Squamous Cell Carcinoma is Associated with decreased Bcl-2/Bax expression ratio and increased apoptosisHuman Pathology 1999 30(9):1097-105. [Google Scholar]
[12]. LM Schoelch, AJ Regezi, PN Dekker, I Ng LO, A McMillan, LB Ziober, Cell cycle proteins and development of oral squamous cell carcinoma.Oral Oncology 1999 35:333-42. [Google Scholar]
[13]. CR Jordan, CG Catzavelos, WA Barrett, MP Speight, Differential expression of Bcl-2 and Bax in squamous cell carcinoma of the oral cavityEur J Cancer B Oral Oncol 1996 32:394-400. [Google Scholar]
[14]. MC Solomon, S Carnelio, V Gudattu, Molecular analysis of oral squamous cell carcinoma: A tissue microarray study.International Journal of Cancer 2010 47(2):166-72. [Google Scholar]
[15]. S Staibano, MD Mignona, L Lo Muzio, Overexpression of cyclin D1, bcl-2 and bax proteins proliferating cell nuclear antigen (PCNA) and DNA ploidy in squamous cell carcinoma of the oral cavityHum Pathol 1998 29:1189-94. [Google Scholar]
[16]. A Rahmani, M Alzohairy, AY Babiker, MA Rizvi, HG Elkarimahmad, Clinicopathological significance of PTEN and bcl2 expressions in oral squamous cell carcinoma.Int J Clin Exp Pathol 2012 5(9):965-71. [Google Scholar]
[17]. WM Sudha, S Hemavathy, Role of bcl-2 oncoprotein in oral potentially malignant disorders and squamous cell carcinoma: an immunohistochemical studyIndian J Dent Res. 2011 22(4):520-25. [Google Scholar]
[18]. N Dwivedi, S Chandra, B Kashyap, V Raj, A Agarwal, Suprabasal expression of Ki-67 as a marker for the severity of oral epithelial dysplasia and oral squamous cell carcinoma.Contemp Clin Dent 2013 4(1):7-12. [Google Scholar]
[19]. T Dadfarnia, BS Mohammed, MA Eltorky, Significance of Ki-67 and p53 immunoexpression in the differential diagnosis of oral necrotizing sialometaplasia and squamous cell carcinoma.Ann Diagn Pathol 2012 16(3):171-76. [Google Scholar]