Influence of Deep Breathing on Heart Rate Variability in Parkinson’s Disease: Co-relation with Severity of Disease and Non-Motor Symptom Scale Score
Mukta Pritam Bidikar1, Gayatri J Jagtap2, Rahul T Chakor3
1 Assistant Professor, Department of Physiology, Topiwala National Medical College & BYL Nair Charitable Hospital, Mumbai, India.
2 Resident, Department of Physiology, Topiwala National Medical College & BYL Nair Charitable Hospital, Mumbai, India.
3 Associate Professor & In-charge Department of Neurology, Topiwala National Medical College & BYL Nair Charitable Hospital, Mumbai, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr Mukta Pritam Bidikar, 15, Kanchan Mrig, Jeevan Vikas Marg, Koldongi, Andheri East, Mumbai-69, India.
Phone: 9821734441,
E-mail: bidikarmukta@gmail.com
Context: Dysautonomia and non-motor symptoms (NMS) in Parkinson’s disease (PD) are frequent, disabling and reduce quality of life of patient.
Aims and Objective: There is a paucity of studies on autonomic dysfunction in PD in Indian population. The study aimed to evaluate autonomic dysfunction in PD patients and co-relate the findings with severity of PD and Non-Motor Symptoms Scale (NMSS) score.
Materials and Methods: We evaluated autonomic function in 30 diagnosed patients of PD (age 55-70 years) and 30 healthy age-matched controls by 3 min deep breathing test (DBT). NMSS was used to identify non-motor symptoms and Hoehn and Yahr (HY) Scale to grade severity of PD. The DBT findings were co-related with severity of PD (HY staging) and NMSS score.
Results: DBT was found to be abnormal in 40% while it was on borderline in 33.3% of PD patients. There was a statistically significant difference (p<0.01) between patients and control group for the DBT. NMS were reported across all the stages of PD but with variable frequency and severity for individual symptom. A negative co-relation was found between results of deep breathing test and clinical severity of disease and NMSS score.
Conclusion: Abnormalities of autonomic function and NMS were integral and present across all the stages of PD patients. Early recognition and treatment of these may decrease morbidity and improve quality of life of PD patients.
Autonomic dysfunction, Indian population, Lewy bodies, NMSS, Parkinson’s disease
Introduction
Parkinson’s disease (PD) is a common, progressive neurodegenerative disorder characterised by a variety of motor and non-motor features. First described by James Parkinson in 1817, PD remains one of the most important disabling motor disorders of later life [1].There are four cardinal features of PD: tremors at rest, rigidity, akinesia (or bradykinesia) and postural instability. Degeneration of neurons in the substantia nigra (pars compacta) and the consequent dysfunction of the dopaminergic nigrostriatal pathway with additional involvement of serotonergic, noradrenergic and cholinergic pathways have been implicated in pathogenesis of PD [2].The type and severity of symptoms in PD may also vary with each individual and the stage of PD.
The CNS controls the heart rate by varying the impulse traffic in sympathetic and parasympathetic limbs of autonomic nervous system (ANS) innervating the S.A node. Variability in the heart rate by physiological stressors such as DBT is a simple and powerful tool in assessment of the parasympathetic limb of autonomic functions [3]. ANS dysfunction in PD although established by previous studies there are contradictory reports and variable results when autonomic dysfunction was evaluated by different methods [4–7]. Previous studies have co-related autonomic dysfunction with age, sex, duration or severity of disease or various autonomic symptoms. Poor autonomic responsiveness has been shown to be associated with advanced Hoehn and Yahr (HY) stage and duration of disease [8].
It has been recognized that non-motor symptoms (NMS) in Parkinson’s disease are also frequently seen in the clinical spectrum of the disease and include dysfunction of the ANS as well. It may consist of various dimensions ranging from cardiovascular, sleep/fatigue, mood/cognition, perceptual problems, attention/memory, gastrointestinal, urinary, sexual functions and miscellany [9]. The 30-item Non-Motor Symptom Scale (NMSS) developed by Chaudhari et al., is a comprehensive scale covering nine domains and provides reliable assessment of NMS in PD [10].
Recent evidence suggests autonomic dysfunction and NMS in PD may occur even in early stage of the disease and may even precede the onset of motor symptoms [11]. Deposition of Lewy bodies in central autonomic regulatory areas such as hypothalamus as well as preganglionic sympathetic and parasympathetic neurons, paravertebral autonomic ganglia has been implicated in the pathogenesis of autonomic dysfunction [12]. The autonomic dysfunction and non-motor symptoms in particular affect the quality of life, hospitalisation rates and health economics in Parkinson’s disease. There is a paucity of studies evaluating autonomic dysfunction in PD in the Indian population. The primary aim of the study was to measure risk of cardiovascular autonomic dysfunction by DBT and NMSS score in 30 PD patients. We also co-related autonomic dysfunction with severity of disease and NMSS score which is an easily quantifiable scale.
Materials and Methods
Patients and Controls: Thirty patients of Idiopathic Parkinson’s disease (age 55-70 years) with duration of disease 7+3 years and fulfilling Parkinson’s Disease Society Brain Bank Clinical Diagnostic Criteria were selected from neurology outpatient department with prior approval of the institutional ethical committee [13]. Patients without any concomitant cardiovascular disease as confirmed by physical examination and normal 12-lead ECG were included in the study. Thirty age matched healthy controls were drawn from family members of patient and from hospital staff. Informed consent of patients and controls was taken. All patients were classified using HY scale to assess the severity of the disease [14]. Also demented patients (as evaluated by mini-mental scale) i.e. with significant cognitive impairment that affected their ability to provide consent and reliable self report were excluded [15]. NMSS score was recorded by standardised NMSS questionnaire [10]. Medications were used alone or in combination: Levodopa alone in 16 patients; levodopa with amantidine in 13 patients and levodopa with bromocriptine in One patient.
Autonomic Test
Deep breathing test: The test was undertaken between 9:00 and 11.00 a.m. in a noise proof and temperature controlled room with patients in their “on” phase. The participants were requested to avoid smoking or caffeine containing beverages for 6h prior to the test. ECG was recorded after the patient had been in supine position for 5min and connected to the standard electrocardiogram (ECG) leads for basal heart rate. Before beginning the test, patients were directed to breathe at a rate of six respiration cycles per minute: 5s for each inhalation and 5s for each exhalation [16]. The ECG was recorded at a speed of 25 mm/sec for 30s while the patient breathed as instructed. The maximum and minimum R-R intervals during each breathing cycle were measured. The result was expressed as the mean of the difference between maximum and minimum heart rates for six measured cycles in beats per minute [17].
Statistical Analysis
Data was analysed using SPSS version 17. Unpaired t-test was used for comparing heart rate variability by deep breathing test of PD patients and controls. Pearson’s correlation was used to correlate heart rate variability and NMSS score and severity of Parkinson disease.
Result
At the baseline mean heart rate (HR) in patients (74.53+4.48 beats/min.) was not significantly different from controls (75.53+3.77 beats/min.). According to the results of the study HRV during deep breathing test (maximum-minimum heart rate) in PD patients was 12.53+ 4.569 which was significantly lower (p<0.05) when compared with controls. The results of DBT were abnormal in 40% of patients, borderline in 33.3% and normal in 26.6% of PD patients. There was no significant difference between the control and PD groups in mean maximum HR however; the control group had a significantly lower mean minimal inspiratory HR [Table/Fig-1].
Comparison of heart rate variability by 3 min deep breathing test in PD patients and controls
| Parameter | Control | PD |
|---|
| Basal heart rate | 75.53+ 3.77 | 74.53+ 4.48 NS |
| Maximal expiratory heart rate (beats/min) | 78.26+ 8.20 | 77.26 + 12.82 NS |
| Minimal inspiratory heart rate (beats/min) | 59.9+ 9.95 | 64.2 + 41.26 * |
| Maximum-Minimum heart rate | 18.37+2.236 | 12.53+ 4.569* |
NS: Not significant*: Significant p < 0.05 when compared to controls
Out of the 16 patients who were only on levodopa DBT was found to be abnormal in 37.5% and borderline in 31.25% of patients. DBT was also found to be abnormal in 23.07% and borderline in 38.46% of patients who were on combination of levodopa with amantidine. Normal result for DBT was recorded in PD patient on combination of levodopa with bromocriptine.
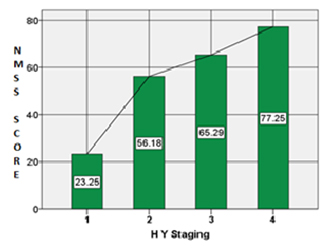
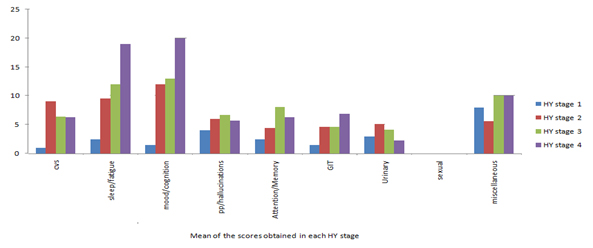
NMS scale consists of nine sub-domains and we obtained a good response for the various domains except for sexual dysfunction. NMS were reported even in stage 1 (HY stages) of PD and a positive co-relation was found between NMSS score and HY staging (r = 0.786) [Table/Fig-2]. NMSS score was also positively correlated with duration of the disease (r= 0.067).When sub-domains of NMS were compared across different HY stages we found cardiovascular symptoms were predominantly present in stage 2 of PD. Symptoms related to sleep, fatigue, mood, cognition and gastrointestinal symptoms however increased with increasing severity of the disease (based on HY stage) [Table/Fig-3].
Co-relation between NMSS Score & HY stage
Sub-domains of NMSS Questionnaire across different Hoehn and Yahr stages.
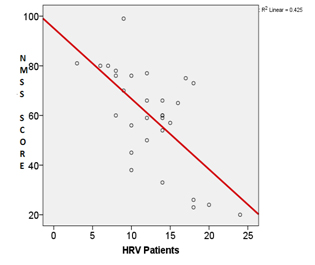
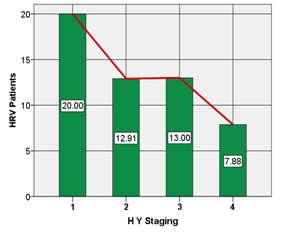
HRV during DBT was negatively correlated with disease duration (r= -0.018), NMSS score (r= -0.652) and HY staging (r= -0.738) [Table/Fig-4,5].
Co-relation between NMSS Score & HRV
Co-relation of HRV with HY staging
Discussion
Heart rate response to deep breathing is considered to be optimal test for cardiovagal function to differentiate normal from neuropathic patients. The changes in heart rate with deep breathing test are primarily due to modulation of cardiac vagal and sympathetic activity and the lung inflation reflex [18]. It has been shown in diabetics that impaired heart rate variation can be an earliest sign of neuropathy and may even precede the autonomic symptoms by several years [19].
Previous studies have shown that spectral analysis of HRV can be useful predictors of autonomic dysfunction in PD patients [20,21]. Few studies done earlier have given contradictory findings for deep breathing test in Parkinson’s disease. In a previous study by Koszewicz M et al., the investigators found that the mean values of heart rate variability percentage at rest and E/I ratio during DBT were significantly lower in PD [22]. Ivanov B et al., evaluated autonomic dysfunction in 40 PD patients by standard tests and found significantly diminished heart rate variability only for DBT [23]. Diminished heart rate variation was found in both recent and advanced PD [24] whereas other investigators did not find significant variation to DBT [25]. In this study we demonstrated that a simple, inexpensive and non-invasive bedside method like deep breathing test to measure HRV can be a sensitive predictor of autonomic dysfunction in PD.
Non-motor features of PD are increasingly being defined and include dysfunction of autonomic nervous system [26]. In the present study various non-motor symptoms were present in PD disease patients with varying frequency and severity except for sexual dysfunction which was not reported by any patients which may be due to cultural reasons. In a previous study by Romenets et al., nocturia, urinary urgency and insomnia were the most prevalent NMS [27]. Khoo TK et al., reported greater NMS in a cohort of PD patients as compared to controls [28]. P Martinez-Martin et al., reported NMS score of 57.1+ 44.0 and higher score with increasing duration of disease and severity [29]. This corroborates with findings of our study.
Autonomic features and NMS are postulated to precede the onset of motor symptoms. This is related with Braak hypothesis suggesting that PD patients have Lewy body pathology in autonomic centers and nerves that include the dorsal motor nucleus of the glossopharyngeal and vagal nerves, gastrointestinal submucosal plexus and postganglionic sympathetic nervous system. These lesions may result in autonomic dysfunction which is present in the premotor stage before nigral involvement [30].
Conclusion
There is a paucity of studies on autonomic dysfunction in PD in Indian population. In the present study we found that parasympathetic autonomic dysfunction and NMS are present even in the early stages of PD. While motor symptoms can be treated effectively the NMS have become major prognostic factor affecting quality of life of PD patients. Early recognition and treatment of these may decrease morbidity and improve prognosis in PD. However, a detailed evaluation of autonomic dysfunction and NMS in a larger sample size would be required to further validate our findings.
NS: Not significant*: Significant p < 0.05 when compared to controls
[1]. Jyh-Gong Gabriel Hou, Eugene C Lai, Michael E. DeBakey. Non motor symptom in Parkinson’s diseaseInternational Journal of Gerontology 2007 1(2):53-64. [Google Scholar]
[2]. Dauer W, Przedborski S, Parkinson’s disease: mechanisms and modelsNeuron 2003 39:889-909. [Google Scholar]
[3]. Shields RW, Heart rate variability with deep breathing as a clinical test of cardiovagal functionCleveland Clinic Journal of Medicine 2009 76(2):S37-S40. [Google Scholar]
[4]. Camerlingo M, Aillon C, Bottacchi E, Gambaro P, D’Alessandro G, Franceschi M, Parasympathetic assessment in Parkinson’s diseaseAdv Neurol 1987 45:267-69. [Google Scholar]
[5]. Turkka JT, Tolonen U, Myllylä VV, Cardiovascular reflexes in Parkinson’s diseaseEur Neurol 1987 26:104-12. [Google Scholar]
[6]. Awerbuch GI, Sandyk R, Autonomic functions in the early stages of Parkinson’s diseaseInt J Neurosci 1992 64(1-4):7-14. [Google Scholar]
[7]. Goetz CG, Lutge W, Tanner CM, Autonomic dysfunction in Parkinson’s diseaseNeurology 1986 36(1):73-75. [Google Scholar]
[8]. van Dijk JG, Haan J, Zwinderman K, Kremer B, van Hilten BJ, Roos RA, Autonomic nervous system dysfunction in Parkinson’s disease: relationships with age, medication, duration, and severityJ Neurol Neurosurg Psychiatry 1993 56(10):1090-95. [Google Scholar]
[9]. Van Rooden SM, M, Visser M, Verbaan D, Marinus J, Van Hilten JJ. Patterns of motor and non-motor features in Parkinson’s diseaseJournal of Neurology, Neurosurgery & Psychiatry 2009 80(8):846-50. [Google Scholar]
[10]. Chaudhuri KR, Martinez-Martin P, Brown RG, The metric properties of a novel non-motor symptoms scale for Parkinson’s disease: Results from an international pilot studyMov Disord 2007 22(13):1901-11. [Google Scholar]
[11]. Asahina M, Vichayanrat E, Low DA, Iodice V, Mathias CJ, Autonomic dysfunction in parkinsonian disorders: assessment and pathophysiologyJournal of Neurology, Neurosurgery & Psychiatry 2013 84(6):674-80. [Google Scholar]
[12]. Stbendorff K, Aarsland D, Minthon L, Londos E, The impact of autonomic dysfunction on survival in patients with Lewy bodies and Parkinson’s disease with dementiaPLOS ONE 2012 7(10):e45451 [Google Scholar]
[13]. Hughes AJ, Daniel SE, Kilford L, Lees AJ, Accuracy of clinical diagnosis of idiopathic Parkinson’s disease. A clinico-pathological study of 100 casesJNNP 1992 55:181-84. [Google Scholar]
[14]. Goetz CG, Poewe W, Rascol O, Sampaio C, Stebbins GT, Counsell C, Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendations the Movement Disorder Society Task Force on rating scales for Parkinson’s diseaseMovement Disorders 2004 19(9):1020-8. [Google Scholar]
[15]. Cummings JL, Mini-mental state examinationJAMA: The Journal of the American Medical Association 1993 269(18):2420-21. [Google Scholar]
[16]. Katz A, Liberty IF, Porath A, Ovsyshcher I, Prystowsky EN, A simple bedside test of 1-minute heart rate variability during deep breathing as a prognostic index after myocardial infarctionAmerican Heart Journal 1999 138(1):32-8. [Google Scholar]
[17]. Ewing DJ, Martyn CN, Young RJ, Clarke BF, The value of cardiovascular autonomic function tests: 10 years experience in diabetesDiabetes Care 1985 8(5):491-98. [Google Scholar]
[18]. Diehl RR, Linden D, Berlit P, Determinants of heart rate variability during deep breathing: basic findings and clinical applicationsClin Auton Res 1997 7(3):131-35. [Google Scholar]
[19]. Watkins PJ, Mackay JD, Cardiac denervation in diabetic neuropathyAnnals of internal medicine 1980 92(2):304-07.Part_2: [Google Scholar]
[20]. Szili-Török T, Dibó G, Kardos A, Paprika D, Rudas L, Abnormal cardiovascular autonomic regulation in Parkinsons diseaseJournal of Clinical and Basic Cardiology 1999 2(2):245-47. [Google Scholar]
[21]. Haapaniemi TH, Pursiainen V, Korpelainen JT, Huikuri HV, Sotaniemi KA, Myllylä VV, Ambulatory ECG and analysis of heart rate variability in Parkinson’s diseaseJournal of Neurology, Neurosurgery & Psychiatry 2001 70(3):305-10. [Google Scholar]
[22]. Koszewicz M, Mendak M, Konopka T, Koziorowska-Gawron E, Budrewicz S, The characteristics of autonomic nervous system disorders in burning mouth syndrome and Parkinson diseaseJ Orofac Pain 2012 26(4):315-20. [Google Scholar]
[23]. Ivanov B, Valkanova V, Deleva N, Cardiovascular autonomic disturbances in early Parkinson’s diseaseAnnual Proceedings IMAB 2004 10:15-8. [Google Scholar]
[24]. Piha SJ, Rinne JO, Rinne UK, Seppänen A, Autonomic dysfunction in recent onset and advanced Parkinson’s diseaseClinical neurology and neurosurgery 1988 90(3):221-26. [Google Scholar]
[25]. De Marinis M, Stocchi F, Gregori B, Accornero N, Sympathetic skin response and cardiovascular autonomic function tests in Parkinson’s disease and multiple system atrophy with autonomic failureMov Disord 2000 15(6):1215-20. [Google Scholar]
[26]. Crosiers D, Pickut B, Theuns J, Paul De Deyn P, Van Broeckhoven C, Martinez-Martin P, Non-motor symptoms in a Flanders-Belgian population of 215 Parkinson’s disease patients as assessed by the Non-Motor Symptoms QuestionnaireAmerican Journal of Neurodegenerative Disease 2012 1(2):160-67. [Google Scholar]
[27]. Rios Romenets S, Wolfson C, Galatas C, Pelletier A, Altman R, Wadup L, Validation of the non-motor symptoms questionnaire (NMS-Quest)Parkinsonism & Related Disorders 2012 18(1):54-8. [Google Scholar]
[28]. Khoo TK, Yarnall AJ, Duncan GW, Coleman S, O’Brien JT, Brooks DJ, The spectrum of nonmotor symptoms in early Parkinson diseaseNeurology 2013 80(3):276-81. [Google Scholar]
[29]. Martinez-Martin P, Rodriguez-Blazquez C, Abe K, International study on the psychometric attributes of the non-motor symptoms scale in Parkinson diseaseNeurology 2009 73(19):1584-91. [Google Scholar]
[30]. Braak H, Del Tredici K, Rub U, Staging of brain pathology related to sporadic Parkinson’s diseaseNeurobiol Aging 2003 24:197-211. [Google Scholar]