Comparison of Microscopic Morphology of Fungi Using Lactophenol Cotton Blue (LPCB), Iodine Glycerol and Congo Red Formaldehyde Staining
Vacharavel Shamly1, Arunava Kali2, Sreenivasan Srirangaraj3, Sivaraman Umadevi4
1Postgraduate, Department of Medical Microbiology, Mahatma Gandhi Medical College & Research Institute, Pondicherry, India.
2Assistant Professor, Department of Microbiology, Mahatma Gandhi Medical College & Research Institute, Pondicherry, India.
3Associate Professor, Department of Microbiology, Mahatma Gandhi Medical College & Research Institute, Pondicherry, India.
4Professor, Department of Microbiology, Mahatma Gandhi Medical College & Research Institute, Pondicherry, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Arunava Kali, Department of Microbiology, Mahatma Gandhi Medical College and Research Institute, Pillaiyarkuppam, Pondicherry – 607402. India.
Phone: 8098439204,
E-mail: ak.arunava@gmail.com
Congo-red formaldehyde, Fungal morphology, Fungal stain, Iodine-glycerol, Lactophenol cotton blue
Sir,
Although fungi were considered as innocuous environmental saprophyte, the significance of fungi as human pathogen is on the raise [1] . Hence, laboratory diagnosis of fungal infections has critical role in patient care. In this prospective study, which was carried out from March to September, 2013, we have evaluated the performance of iodine-glycerol in comparison to lactophenol cotton blue (LPCB) and Congo-red formaldehyde as an alternative method for identification of filamentous fungi.
A total of 180 clinical samples, obtained from skin, hair, nail, sputum, broncho-alveolar lavage, corneal scrapings and tissue of various inpatient and outpatient departments of Mahatma Gandhi Medical College & Research Institute, Pondicherry, India were processed for fungi and included in this study.
Direct microscopic examination was performed using potassium hydroxide (KOH) iodine glycerol and Congo red formaldehyde wet mounts [Table/Fig-1,2and3]. Culture was done in duplicate in SDA with and without cycloheximide at 25oC and 37oC incubation.
Tease mount and slide cultures of 20 standard fungal strains (belonging to the genus Aspergillus, Penicillium, Fusarium, Curvuleria, Mucor, Rhizopus, Syncephalastrum, Trichophyton, Epidermophyton and Microsporum) and fungal clinical isolates grown on culture were stained by Congo red formaldehyde, LPCB and iodine-glycerol in an attempt to compare their staining characteristics.
Iodine glycerol reagent was prepared in-house by addition of glycerol (0.25%) in iodine solution as described by Vignesh et al., [2] . Whereas for Congo red formaldehyde stain, 10% formaldehyde solution was diluted to 1% and Congo red was added to achieve 0.1% final concentration at pH 7.1 [3] . Wet preparations of Congo red formaldehyde were examined under fluorescence microscope. Fungal culture was considered as the gold standard test and sensitivity, specificity, positive predictive value and negative predictive value were calculated.
Total 32 fungal isolates were recovered in culture [Table/Fig-4]. The performance of KOH, iodine glycerol and Congo red formaldehyde wet mounts in comparison to culture are expressed in terms of sensitivity, specificity, positive predictive value and negative predictive value and detailed in [Table/Fig-5]. Direct microscopic detection of fungal pathogens has immense value in routine mycology workup. Especially in case of opportunistic mycoses, isolates grown on culture often require direct microscopic confirmation to rule out the possibility of laboratory contamination [4] . KOH, in various concentrations, has been employed as a keratolytic agent to demonstrate fungi in tissue [5] . However, it does not stain fungal elements. Consequently, delicate semi-transparent fungal elements escape detection [6-8] . On the other hand, collagen fibers, tissue matrix, lipid vesicles, air bubbles, and textile fibers are often difficult to distinguish from fungi [6] . Several authors had reported low sensitivity and specificity of KOH mounts [6-8] . Saxena et al., reported 68% sensitivity and 40% specificity [6] . In our study, the sensitivity and specificity of KOH was 65.6% and 93.9% respectively.
Fluorescent dyes which bind specifically to fungal cell wall have essential role in mycology. Calcofluor white was traditionally used as a fluorescent brightener in paper and textile industries [3,5] , has been extensively used to study cell walls of fungi and bacteria. The use of other fluorescent agents in mycology have been reported infrequently. Congo red is a diazo compound which is commonly used to stain amyloid in tissue sections [3] . Pertaining to its high affinity for cellulose or chitin, Congo red also permits the detection of fungal elements in tissue. Although Congo red is cost-effective compared to Calcofluor white, its use as fungal stain is still unconventional [3,9] . Congo red in conjunction with glutaraldehyde or formaldehyde, as fungicidal agent, had been reported to be useful for rapid detection fungi in both clinical specimens and fungal culture preparations [3] . We found that yeast cells and both septate and asepate hyphae in clinical specimens had bright orange fluorescence. In case of culture preparations of filamentous fungi, the cell walls, cross-walls of hyphae, as well as the hyphal tips and rhizoids were visible. However, the conidia of molds, Aspergillus, Penicillium and Fusarium species in particular, were indistinct and often not visible under fluorescent microscope. Slifkin et al., found good staining result of Congo red for a variety of yeasts and molds except the cell wall of Phialophora species and the capsule of Cryptococcus neoformans which were poorly stained [3] . In another study, Candida and Histoplasma isolates were found Congo red negative unlike Pityrosporum and Blastomyces isolates [9] .
Although KOH and Congo red are easily available and inexpensive reagent, both may have potential health hazard. As per material safety data sheets, formaldehyde used in Congo red preparations is cytotoxic and carcinogenic [10] . Whereas, KOH solutions with concentrations higher than 2% are corrosive [11] . In a recent study, better microscopic morphology of fungi was obtained using iodineglycerol in comparison to LPCB [2] . In iodine-glycerol method, iodine acts as fungicide as well as an agent for staining fungal structures. Glycerol prevents rapid drying of the wet preparation. In contrast to phenol, KOH and formaldehyde, iodine has no potential biohazards [2] . Furthermore, while comparing microscopic morphology of fungi, best results were achieved with LPCB and iodine glycerol. Both preparations had more transparency and high clarity. Thin delicate hyphae and semitransparent spores were visualized clearly which allowed rapid identification. However, we observed that iodine-glycerol and Congo red formaldehyde preparations were unsatisfactory for detection of fungi in clinical samples. The sensitivity of iodine-glycerol and Congo red formaldehyde methods were 59.3% and 43.8% [Table/Fig-5]. This may be due to the inability of these two methods to visualize fungal elements embedded deeply in tissue as keratin and tissue materials were not digested by a keratolytic agent. Furthermore, Congo red formaldehyde method also had limitations of rapid fading of fluorescence on light exposure and drying. As a result, Congo red formaldehyde preparations were not suitable for long term preservation.
In conclusion, Iodine-glycerol preparation was found to be a promising technique for identification fungal isolates which may be employed as a non-hazardous and safer alternative to LPCB for fungal identification.
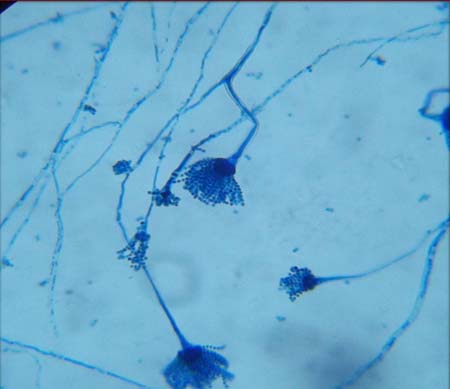
Tease mount preparation of fungal culture using LPCB showing penicillium sp
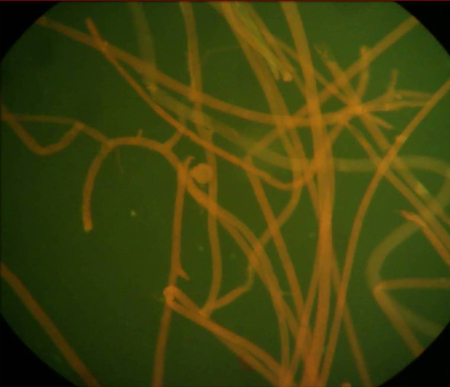
Formalin-Congo red-stained tease mount preparations of zygomyces showing aseptate hyphae
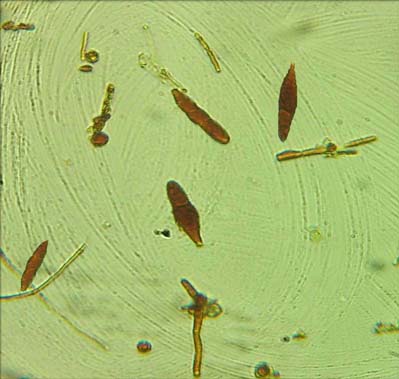
Wet preparation using iodine glycerol showing macroconidia of Microsporum spp
Fungal isolate recovered in culture from clinical samples
| Fungal isolate | Number | Percentage |
| Candida albicans | 8 | 4.4% |
| Non-albicans Candida species | 11 | 6.1% |
| Aspergillus fumigatus | 4 | 2.2% |
| Aspergillus flavus | 2 | 1.1% |
| Aspergillus niger | 1 | 0.5% |
| Penicillium species | 1 | 0.5% |
| Fusarium species | 1 | 0.5% |
| Mucor species | 2 | 1.1% |
| Rhizopus species | 1 | 0.5% |
| Trichophyton mentagrophytes | 1 | 0.5% |
Comparison of three methods for microscopic detection of fungi in clinical samples
| | Sensitivity | Specificity | Positive predictive value | Negative predictive value |
| KOH wet preparation | 65.6% | 93.9% | 93.9% 70% | 92.6% |
| Iodine glycerol wet preparation | 59.3% | 98.6% | 90.4% | 91.8% |
| Congo red formaldehyde wet preparation | 43.8% | 97.9% | 82.3% | 88.9% |
[1]. F Saliba, Emerging fungal infections.Expert Rev Anti Infect Ther 2012 10(4):419-21. [Google Scholar]
[2]. R Vignesh, CR Swathirajan, S Solomon, EM Shankar, KG Murugavel, I Paul, Iodine-glycerol as an alternative to lactophenol cotton blue for identification of fungal elements in clinical laboratoryIndian J Med Microbiol 2013 31(1):93-4. [Google Scholar]
[3]. M Slifkin, R Cumbie, Congo red as a fluorochrome for the rapid detection of fungiJ Clin Microbiol 1988 26(5):827-30. [Google Scholar]
[4]. Koneman’s Color Atlas and textbook of Diagnostic Microbiology 2005 5th EditionUSALippincott Williams and Wilkins Company:547-52. [Google Scholar]
[5]. JG Collee, AG Fraser, BP Marmion, A Simmons, Mackie & McCartney Practical Medical Microbiology.Churchill Livingstone 2010 14th Edition [Google Scholar]
[6]. A Saxena, M Capoor, S Yadav, V Ramesh, Comparison of direct microscopic methods using potassium hydroxide, periodic acid Schiff, and calcofluor white with culture in the diagnosis of onychomycosis.Indian J Dermatol Venereol Leprol 2013 79(2):242-3. [Google Scholar]
[7]. MA Lawry, E Haneke, K Strobeck, S Martin, B Zimmer, PS Romano, Methods for diagnosing onychomycosis: a comparative study and review of the literatureArch Dermatol 2000 136(9):1112-6. [Google Scholar]
[8]. V Panasiti, RG Borroni, V Devirgiliis, M Rossi, L Fabbrizio, R Masciangelo, Comparison of diagnostic methods in the diagnosis of dermatomycoses and onychomycosesMycoses. 2006 49(1):26-9. [Google Scholar]
[9]. GK Axelson, T Giorgadze, GA Youngberg, Evaluation of the use of Congo red staining in the differential diagnosis of Candida vs. various other yeast-form fungal organisms.J Cutan Pathol 2008 35(1):27-30. [Google Scholar]
[10]. IPCS. Formaldehyde: Health and Safety Guide. Geneva: WHO; 1991 [cited 2014 Jan 12]; Available from: http://www.inchem.org/documents/hsg/hsg/hsg057. htm. [Google Scholar]
[11]. OECD SIDS. Potassium hydroxide. Switzerland: UNEP Publications; 2001 [cited 2014 Jan 12]; Available from:http://www.inchem.org/documents/sids/sids/ POTASSIUMHYD.pdf. [Google Scholar]