Introduction
Antimicrobial resistance (AMR) including multidrug resistance (MDR), is on the rise among many microorganisms in health-care facilities as well as in community [1]. Reported data suggests that almost 2 million cases of infection with resistant bacteria have been reported in the United States (US) every year leading to $20 billion incremental direct healthcare cost [2]. Estimates of European Medicines Agency (EMA) and European Centre for Disease Prevention and Control (ECDC) reported a toll of 25,000 deaths per year as a direct consequence of a MDR infection with total cost of €1.5 billion [3]. In Canada, hospitalization caused by resistant infections resulted in higher economic burden with excess cost of $9–$14 million [1]. Study by Indian Network for Surveillance of Antimicrobial Resistance (INSAR) group, India reported prevalence of 41% with methicillin resistant Staphylococcus aureus (MRSA) [4]. High prevalence of Gram–negative bacterial resistance has also been reported in India [5]. World Health Organization (WHO) estimates that worldwide 3.7% of new cases and 20% of previously treated cases are estimated to have MDR-TB [6].
The path of antibiotic development is challenged at every step by the emerging microbial resistance. Emergence of MRSA, resistant Pseudomonas aeruginosa have already compromised the most effective treatments [7]. Recent reports have witnessed changing susceptibility pattern and spreading trend in AMR [8–10].Development of superbug like New Delhi metallo-β-lactamase 1 (NDM-1) positive Enterobacteriaceae have further complicated the management of such infections [11].
Urgent threats with Clostridium difficile, carbapenem-resistant Enterobacteriaceae (CRE) and drug-resistant Neisseria gonorrhoeae have been reported by US Centers for Disease Control and Prevention (CDC) [2]. Thus AMR is a major point of concern as it is associated with high death rates and fear of progression to the pre antibiotic era; also has potential to hamper infectious disease control programmes; increase in health care costs and diminish health-care gains achieved so far [6].This review discusses various measures that can be taken to combat AMR with major emphasis on antibiotics resistance.
Call for action
The global threat of AMR calls for the collaborative action for developing effective strategies in combating AMR. CDC recommends 12 steps to prevent antimicrobial resistance in a healthcare setting [12].
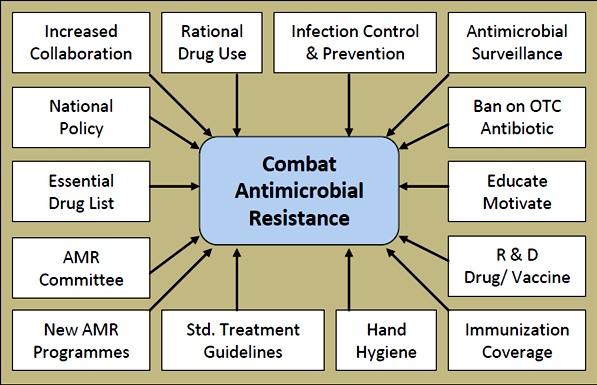
We herein discuss the effective strategies for combating AMR. The collaborative approach is required at different levels. Different measures for combating AMR can be directed at international, national, community, hospital, individual and patient level. [Table/Fig-1] summarizes these approaches.
Approaches for combating of antimicrobial resistance
Std.: Standard, R & D Research and Development, OTC: Over-the-Counter
1. International Measures
As a global problem, AMR is now well accepted by various stakeholders. In the year 2011, WHO theme was on combating antimicrobial resistance. It was one of the major attempts to draw international attention and need of combined efforts to alleviate the problem of AMR [13]. Some of the WHO recommended approaches are listed below: [14]
Increased collaboration between governments, nongovernmental organizations, professional groups and international agencies
New networks that undertake surveillance of antimicrobial use and AMR
International approach for control of counterfeit antimicrobials
Incentives for the research and development of new drugs and vaccines
Forming new, and reinforcing existing programmes to contain AMR.
2. National Strategies
2.1 National committee with intersectoral coordination and regulatory actions
Establishment of national committee to monitor impact of antibiotic resistance and provide intersectoral co-ordination is required. WHO recommends that such committee would formulate AMR policy; provide guidance on standards, regulations, training and awareness on antibiotic use and AMR. Developing indicators to monitor and evaluate the impact of AMR prevention and control strategies would be amongst priority objectives at national level [15].Further WHO advises that having a registration scheme for all dispensing outlets, making prescription-only availability of antimicrobials, legal binding on all manufacturers to report data on antimicrobial distribution and incentives for rational use of antimicrobials can help contain AMR [15].
Establishing and implementing national standard treatment guidelines, having essential drug list (EDL), enhancing coverage of immunization are other essential strategies desired at national level [14].
2.2 National Antimicrobial Resistance Policy, India
A national policy for containment of AMR was introduced in 2011. The policy aims to understand emergence, spread and factors influencing AMR, to setup antimicrobial program, to rationalize the use of antimicrobials and to encourage the innovation of newer effective antimicrobials. In addition, some major action points identified in national policy were; establishing AMR surveillance system, strengthening infection prevention and control measures and educate, train and motivate all stake holders in rational use of antimicrobials [16]. WHO estimates that less than 50% of all countries are implementing basic policies for appropriate use of medications [17].
3. Action at Community Level
Globally, infectious diseases still continue to be significant cause of morbidity and mortality, affecting more the countries where health services are not sufficiently accessible [18]. Kardas et al., in a review of antibiotic misuse in the community reported that at community level, more than one third of patients were non-compliant to the antibiotic regimen and one quarter kept the unused antibiotics for use in future [19]. This indicates a poor antibiotic-taking behavior [19]. Review on population perspective of AMR by Lipsitch et al., suggests that prevention of AMR in an individual suffering from infection is one of the basic method to prevent further spread of resistance to the wider community [20]. The increasing rate of resistance among community acquired infections like upper and lower respiratory tract infections, bacterial diarrhea, typhoid fever are not matched by development of newer antibiotics [21]. Thus there is urgent need for reforms at community level for curtailing AMR. Different measures directed to control and prevent AMR at community levels are the need of an hour.
3.1 Rational use of antibiotics
Irrational use of medicines is a serious global problem. In developing countries, at primary level, less than 40% patients in public sector and less than 30% patients in private sector are treated in accordance with standard treatment guidelines [17].This mandates public and professional education towards rational use of antibiotics.
3.2 Over-the-counter (OTC) antibiotics
Measures that preserve efficacy of antimicrobials are mainly directed towards the hospitals and drug providers and missing antibiotic use without prescription. In systematic review of non-prescription antimicrobial use, Morgan et al., reported that non-prescription use of antimicrobials varied from as low as 3% in northern Europe studies to 100% in African studies [22]. This implies urgent need for regulatory control on OTC use of antibiotics.
3.3 Guidelines for use of antibiotics at local levels
About use of antibiotics in common situations, Bhagwati A. discussed that an empirical antibiotic therapy should be started considering the clinical condition of the patient and prevalent pathogen and resistance pattern in a locality. Appropriate change in antibiotic is required as per the sensitivity of microbe. Antibiotic guidelines are therefore must to optimize antibiotic selection with their dosing, route of administration and duration of therapy [21].
3.4 Standards of hygiene
Use of alcohol-based hand rubs or washing hands has proven efficacy in prevention of infection [12,23]. This factor can restrict the spread of infection and thereby the AMR. Willingness to put up with high standards of hygiene is the need of an hour.
3.5 Other approaches
These include identifying residents with MDR infections and use of standard treatment regime for their management, vaccination, infection prevention strategies and ban on OTC sale of antimicrobials [12,16].
4. At Hospital or health care setting
A person or a patient in a health care facility is at higher risk of infection with common pathogens. For control and containment of AMR, experts recommend some of the measures as discussed herein.
4.1 Infection prevention and control within health-care facilities [1,24]
Infection prevention and control measures are designed to reduce the spread of pathogens including resistant ones within healthcare facilities and to the wider community. This can prevent further infections and AMR spread [1]. Recommended measures to prevent and control infection in a health-care facility [1,12,16,24–26].
Establishing an infection prevention and control committee (IPC).
Good hand hygiene practices.
Effective diagnosis and treatment of infection.
Rational antimicrobial use.
Surveillance of antibiotic resistance and antibiotic use.
Improving the antimicrobial quality and supply chain.
Good Microbiology Practices.
Surveillance of Antibiotic resistance and antibiotic use
All over the world, surveillance is considered as strength of the programmes directed towards AMR. The objective of surveillance is to facilitate the containment of antibiotic resistance. It is a useful tool that generates data on antimicrobial use and AMR which is essential in updating national EDLs and formulating infection control policies. It may also help in improving antimicrobial prescribing and development of empirical therapy or standards treatment guidelines [25,26].National policy on AMR in India recommend three types of surveillances which include comprehensive surveillance, sentinel surveillance, and point prevalence [16].
Good Microbiology Practices
From accurate collection, handling of specimens to the speedy reporting with standard microbiology practices may help in prevention of AMR spread. Testing with international standards, reporting of resistance pattern to IPC and monitoring the sterilization and disinfection activities underlie the good microbiology practices [16,24].
5. At Personal / Patient level
5.1 Role of Physician
Along with providing direct patient care, complying with local infection control and antibiotic use policies and timely notifying resistant cases to IPC, the physician can play a major role in combating AMR [24]. Identifying and preventing situations that may act as nidus for infection may help curtail unnecessary infections and thereby AMR [12].
5.2 Role of Nurses/health care providers
Since nurses/health care providers are in direct contact with the patients, they are amongst those related in either spread or control of infection and AMR. Educating nurses and health care providers about the AMR and aseptic practices may help in control of spread of infections. Moongtui et al., have reviewed the role of nurses in preventing AMR and reported the initiatives by Thailand like having Master’s programme in infection control nursing with other short training courses and involvement of nurses in AMR prevention and control programme [27].
5.3 Role of Pharmacist
McCoy et al., in their review discussed the Pharmacist-directed antibiotic stewardship programs (ASPs) as an approach to improve the utilization of antibiotics. Pharmacists can counsel patients with viral infections about the ineffectiveness of antibacterials and can recommend appropriate OTC medication for supportive care. Referral to physician is must if a bacterial infection is suspected. Above all, most importantly, addressing patient and clinician concerns related to antimicrobial and understanding of the appropriate use of these agents, pharmacist can be an essential arm in preventing AMR [28]. Pharmacist is the key person to educate patients about antimicrobial use and the importance of complying with the prescribed treatment regime. This may help to reduce the unnecessary use of antibiotics.
5.4 At patient level
(a) Aseptic protocol for any procedures.
Parameswaran et al., reported that MDR microbes caused 30.2% of the catheter-related blood-stream infections and empirical treatment had no role in prevention of such infections [29]. This mandates use of aseptic protocol to minimize local or systemic infections associated with any procedures.
(b) Breaking the chain of infectivity [12].
By simple means like covering mouth while coughing or sneezing, infection spread can be reduced.
(c) Compliance to the antimicrobial regime and antibiotic.
Improved compliance definitely can improve the rate of infection control. Patient education on compliance with antibiotics is must [30]. Using established regimes for prophylactic use of antibiotics in high risk cases and for the shortest duration possible can minimize risk of AMR [31].
6. Other Measures
6.1 Pharmaceutical promotion
WHO recommends that pharmaceutical firms should strictly adhere to the standards of drug promotion, direct-to-consumer advertising and advertising the internet [14]. There is need to identify and prohibit any incentives promoted by pharmaceutical companies that possibly encourage inappropriate antimicrobial use.
6.2 Antibiotic use in animals
Use of antibiotic avoparcin in food of the animals in Europe was the cause of development of vancomycin-Resistant Enterococci (VRE) and consequent colonization in human intestine, thus highlighting its importance [16]. WHO specifically called for stricter legislation to minimize antimicrobial usage in animals. Improved sanitation, provision of probitotics or nutritional supplements in feed and vaccination for common animal diseases can help reduce the antimicrobial use in animals [25].
6.3 Rapid understanding of the AMR mechanisms
In their review, Bergeron and Ouellette suggested that genotyping of bacteria and identification of resistant genes in bacteria can impact the treatment of infections and contribute to the control of AMR [32].
6.4 Innovation in new drugs and technology
Concerns of increased antibiotic resistance lead to the urgent need of concentrating on the issue of new drugs and vaccines development to combat AMR. Collaborated efforts of national, international, government and academic networks are needed to identify new classes of antibiotics and diagnostic technologies [15].Providing research funding for development of new antimicrobials to pharmaceutical companies for diseases of public health importance can advance the new drug development.
In summary, it is necessary to enforce essential actions to be taken by government to inspire change by all stakeholders related to AMR as described in WHO policy package for combating AMR [13]. This policy package refers to:
Dedicate to a comprehensive, financed national plan with accountability of each one involved and engagement of civil societies
Improve and strengthen surveillance and laboratory capacity and facilities
Make sure uninterrupted wide access to essential medicines of assured quality
Regulate and encourage rational use of medicines, even in animal husbandry, and ensure proper patient care
Improvise on infection prevention and control
Promote and pursue innovations and research and development for new tools
Conclusion
Antimicrobial resistance is a complex problem with many diverse contributing factors. It is major cause of health concerns adding cost to oneself and to the community, directly or indirectly. Prevention is still the best tool to reduce the infection spread and thereby AMR. Along with rational use of existing antimicrobial drugs, development of new effective compounds and new diagnostic technology is the need. Joint efforts from patients, prescribers and individuals to international regulators and policy makers are needed to fight against the globally spreading antimicrobial resistance.
[1]. The evolving threat of antimicrobial resistance. Options for action. World Health Organization, 2012 [Google Scholar]
[2]. Antibiotic Resistance Threats in the United States, US Department of Human and Health Services, Centre for Disease Control and prevention, 23, 2013 [Google Scholar]
[3]. ECDC/EMEA Joint Technical ReportThe bacterial challenge: time to react. European Centre for Disease Prevention and Control, 2009EMEAdoc. ref. EMEA/576176/2009 [Google Scholar]
[4]. Methicillin resistant Staphylococcus aureus (MRSA) in India: Prevalence & susceptibility pattern. Indian Network for Surveillance of Antimicrobial Resistance (INSAR) group, IndiaIndian J Med Res 2013 137:363-9. [Google Scholar]
[5]. Ghafur A, Mathai D, Muruganathan A, Jayalal JA, Kant R, Chaudhary D, The Chennai Declaration Recommendations of A roadmap to tackle the challenge of antimicrobial resistance - A joint meeting of medical societies of IndiaIndian J Cancer[Epub ahead of print] [Cited 2013 Oct 21]. Available from: http://www.indianjcancer.com/preprintarticle.asp?id=104065 [Google Scholar]
[6]. Antimicrobial resistance. WHO fact sheet, Fact sheet N°194. Updated May 2013. Accessed on 17. 2013 [Google Scholar]
[7]. Davies J, Davies D, Origin and evolution of antibiotic resistanceMicrobiology and Molecular Biology Reviews 2010 74(3):417-33. [Google Scholar]
[8]. Abhilash KPP, Veeraraghavan B, Abraham OC, Epidemiology and Outcome of Bacteremia Caused by Extended Spectrum Beta-Lactamase (ESBL)-producing Escherichia Coli and Klebsiella Spp. in a Tertiary Care Teaching Hospital in South IndiaJAPI (supplement) 2010 58:13-17. [Google Scholar]
[9]. Patel PH, Rathod S, Chuahan B, Rathod H, Pethani J, Shah P, Changing Trend of Antibiotic Susceptibility Pattern of Common Gram Negative Bacilli Isolated From Medical Intensive Care Unit of Tertiary Care Hospital Ahmedabad, Gujarat, IndiaJournal of Drug Discovery and Therapeutics 2013 1(4):16-20. [Google Scholar]
[10]. Deshpande VR, Karmarkar MG, Mehta PR, Prevalence of multidrug-resistant enterococci in a tertiary care hospital in Mumbai, IndiaJ Infect Dev Ctries 2013 7(2):155-58. [Google Scholar]
[11]. Nazir T, Abraham S, Islam A, Emergence of Potential Superbug Mycobacterium Tuberculosis, Lessons from New Delhi Mutant-1 Bacterial StrainsInternational Journal of Health Science 2012 6(1):87-94. [Google Scholar]
[12]. CDC Campaign to Prevent Antimicrobial Resistance in Healthcare Settings, 12 Steps to Prevent Antimicrobial Resistance among Long-term Care Residents. Department of Health and Human Services Centers for Disease Control and Prevention. March 2004. Accessed on 10. 2013 [Google Scholar]
[13]. Leung E, Weil DE, Raviglione M, Nakatani H, World Health Organization. World Health Day Antimicrobial Resistance Technical Working Group. The WHO policy package to combat antimicrobial resistanceBull World Health Organ 2011 89(5):390-2. [Google Scholar]
[14]. WHO Global Strategy for Containment of Antimicrobial Resistance, World Health Organization 2001. WHO/CDS/CSR/DRS/2001.2 [Google Scholar]
[15]. European strategic action plan on antibiotic resistance, Regional Committee for Europe, EUR/RC61/14 EUR/RC61/Conf.Doc./7, World Health Organization Regional Office for Europe, June 2011 [Google Scholar]
[16]. National Policy for Containment of Antimicrobial Resistance, Directorate General of Health Services, Ministry of Health & Family Welfare, India 2011 [Google Scholar]
[17]. Holloway K, Dijk L, Rational Use of Medicines, The World Medicines Situation 2011 2011 3rd EditionWorld Health OrganizationWHO/EMP/MIE/2011.2 [Google Scholar]
[18]. Raghunath D, Emerging antibiotic resistance in bacteria with special reference to IndiaJ Biosci 2008 33(4):593-603. [Google Scholar]
[19]. Kardas P, Devine S, Golembesky A, Roberts C, A systematic review and meta-analysis of misuse of antibiotic therapies in the communityInternational Journal of Antimicrobial Agents 2005 26(2):106-13. [Google Scholar]
[20]. Lipsitch M, Samore MH, Antimicrobial use and antimicrobial resistance: A population perspectiveEmerging Infectious Diseases 2002 8(4):347-54. [Google Scholar]
[21]. Bhagwati A, Guidelines for antibiotic usage in common situationsJAPI (supplement) 2010 58:49-50. [Google Scholar]
[22]. Morgan DJ, Okeke IN, Laxminarayan R, Perencevich EN, Weisenberg S, Non-prescription antimicrobial use worldwide: a systematic review. NIH Public Access, Author ManuscriptLancet Infect DisAuthor manuscript; available in PMC 2013 January 14. Accessed on 24 Oct 2013. [Published in final edited form as: Lancet Infect Dis. 2011 September; 11(9): 692–701.] [Google Scholar]
[23]. WHO Guidelines on Hand Hygiene in Health Care. World Alliance for patient safety, World Health Organization, 2009 [Google Scholar]
[24]. Prevention of hospital-acquired infections. A practical guide. 2nd edition. World Health Organization 2002. WHO/CDS/CSR/EPH/2002.12 [Google Scholar]
[25]. Ganguly NK, Arora NK, Chandy SJ, Fairoze MN, Gill JPS, Gupta U, Rationalizing antibiotic use to limit antibiotic resistance in India. Global Antibiotic Resistance Partnership (GARP) – India Working GroupIndian J Med Res 2011 134:281-94. [Google Scholar]
[26]. Essack SY, Strategies for the Prevention and Containment of Antibiotic ResistanceSA Fam Pract 2006 48(1):51 [Google Scholar]
[27]. Moongtui W, Picheansathian W, Senaratana W, Role of nurses in prevention of antimicrobial resistanceRegional Health Forum 2011 15(1):104-11. [Google Scholar]
[28]. McCoy D, Toussaint K, Gallagher JC, The Pharmacist’s Role in Preventing Antibiotic ResistanceUS Pharm 2011 36(7):42-49. [Google Scholar]
[29]. Parameswaran R, Sherchan JB, Varma D M, Mukhopadhyay C, Vidyasagar S, Intravascular catheter-related infections in an Indian tertiary care hospitalJ Infect Dev Ctries 2011 5(6):452-58. [Google Scholar]
[30]. Kardas P, Patient compliance with antibiotic treatment for respiratory tract infectionsJournal of Antimicrobial Chemotherapy 2002 49:897-903. [Google Scholar]
[31]. Bratzler DW, Dellinger EP, Olsen KM, Peri TM, Auwaerter PG, Bolon MK, Clinical practice guidelines for antimicrobial prophylaxis in surgery. American Society of Health-System Pharmacists (ASHP) reportAm J Health-Syst Pharm 2013 70:195-283. [Google Scholar]
[32]. Bergeron MG, Ouellette M, Preventing Antibiotic Resistance through Rapid Genotypic Identification of Bacteria and of Their Antibiotic Resistance Genes in the Clinical Microbiology LaboratoryJournal of Clinical Microbiology 1998 36(8):2169-72. [Google Scholar]