Introduction
Urolithiasis is the third most common disorder of the urinary tract, is defined as the formation of sediment in the urinary tract consisting of one or more of the poorly soluble crystalloids of urine. It is a worldwide problem particularly common in parts of United States, South Africa, India and South East Asia. Approximately 2% of the world population experiences renal stone disease with a male-female ratio of 2:1 and the peak incidence is observed in 2nd to 3rd decade of life. Renal calculi are characterised clinically by colicky pain (renal colic) as manifest by hematuria. Major risk factors responsible for the nephrolithiasis are inadequate urinary drainage, microbial infections, diet with excess oxalates and calcium, vitamin abnormalities i.e.; deficiency of Vitamin-A, excess of vitamin D, metabolic diseases like hyperparathyroidism, cystinuria, gout, intestinal dysfunction [1] and environmental factors like hot and dry climatic conditions [2]. Despite various advanced and numerous methods available for the treatment of urolithiasis in the allopathic system of medicine, it suffers from few disadvantages that force the patients to go for other forms of medicine like Ayurveda, Homeopathy, Unani and Folklore medicine etc. A vast number of medicinal plants mentioned in ayurvedic system of medicine are known to possess antiurolithic properties i.e; Didymocarpus pedicellata, Saxifraga ligulata, Rubia cordifolia, Cyperus scariosus, Achyranthes aspera, Cissampelos pareira, Onosma bracteatrum, Veronica cinerea and herbomineral preparations Shilajeet and Hajrul yahood bhasmas etc.
Plant Description
The Cissampelos pareira [3], an extensively spreading, glabrous to soft pubescent, perennial climbing shrub found all over India Sectionand is commonly known as Padha and other synonyms are Padvel, Padvali, Aaknadi, Venievel, Poda and Patha belongs to the family of Menispermaceae [3]. In Ayurvedic system of medicine, the leaves and roots are used in the treatment of indolent ulcers (Kirtikar and Basu,) and diarrhea (Amresh et al.,). The plant is used in the treatment of urinary tract infections since it is considered as antiseptic (Dandiya and Chopra,). Juice of C. pareira is given in migraine and the plant has a long history of use for inflammation of muscles, snakebite, rheumatism, diarrhea, dysentery and menstrual problems. C. pariera is widely employed in herbal medicine today as a diuretic, tonic as well as to reduce fever and to relieve pain. It is often employed for menstrual cramps, dysmenorrhoea, excessive bleeding and uterine hemorrhages, fibroid tumours, pre and post natal pain, colic, constipation, poor digestion and dyspepsia. Some scientific studies revealed its antinociceptive [4], antiarthritic [4], cardiotonic [5], anticancer [6], anti-inflammatory [7], antidiarrheal [8], anti-hemorrhagic, antifertility [9], antioxidant, neuroprotective [10], hepatoprotective [11], antioxidant [12], immunomodulatory [12], anti trypanosomal activities. The major constituents of roots of C.pareira include [13] Pelosin, O-methylcurine, l-curine Cissamine, Cissampareine, Hyatin, Bebeerine, Cycleanine, Tetrandine and Beriberine, Cissampeline, Cissampoline, Dicentrine, Insularine, Pareirine, Hyatinine, Pareirubrine A, Pareirubrine B, Pareitropone, Norimeluteine, Cissampeloflavone, D-Quercitol and Grandirubrine [13]. The roots of C.pareira are traditionally used as an antiurolithic but scientifically not evaluated as a antiurolithic agent. The main aim of the present study was to evaluate antiurolithic activity of roots of C. pareira in 2% Ammonium chloride (AC) and 0.75% Ethylene glycol (EG) induced urolithiasis in albino rats.
Methodology
Collection of Plant
The roots of C. pareira were collected from the forest of Tirupati, AP and were identified and authenticated by Pharmacognocist V.L.College of Pharmacy, Raichur, Karnataka, India.
Preparation of Extract
Roots thoroughly washed under tap water and dried under shade powdered by using a mechanical grinder. The alcoholic extract of roots of C. Pareira was prepared by soxhletation. About 200 g of root powder was taken into the soxhlet apparatus and extracted using (95%) ethanol. The extraction process was carried out for 18-20 h till the appearance of colourless solvent in the side tube. The extract collected was dried by evaporating the solvents on a water bath maintained at <500 C and percentage yield of alcoholic extract of roots of C. pareira was recorded with respect to the total quantity of powder used for the extraction. Then the extract was evaluated for its phytochemicals by following standard procedures.
Preparation of Extract
Roots thoroughly washed under tap water and dried under shade powdered by using a mechanical grinder. The alcoholic extract of roots of C. Pareira was prepared by soxhletation. About 200 g of root powder was taken into the soxhlet apparatus and extracted using (95%) ethanol. The extraction process was carried out for 18-20 h till the appearance of colourless solvent in the side tube. The extract collected was dried by evaporating the solvents on a water bath maintained at <500 C and percentage yield of alcoholic extract of roots of C. pareira was recorded with respect to the total quantity of powder used for the extraction. Then the extract was evaluated for its phytochemicals by following standard procedures.
Experimental Animals
Male Albino rats (54) weighing between 140-200 g used in the study (9 Groups; n = 6) were obtained from the Central Animal House, V.L.College of Pharmacy, Raichur, Karnataka, India. The experimental protocol was approved by the Institutional Animal Ethical Committee. The animals were maintained under standard husbandry conditions temperature 22+20C, humidity 45-55%, light : dark cycle (12:12h) for an acclimatization period of 15 days before performing the experiments. All rats were placed in metallic cages, three in each.
Ethics
The experiments compiled with the guidelines for animal experimentation of our laboratory and was approved by the Institutional Animal Ethical Committee, VLCP, Raichur.
Drugs and Chemicals Used
Cystone 5 ml/kg (Himalaya drug company, Bangalore, India.), Ethylene glycol (S.D Fine chemicals, Hyderabad, Andhra Pradesh, India), Ammonium chloride (S.D Fine chemicals, Hyderabad, Andhra Pradesh, India), CMC (S.D Fine chemicals, Hyderabad, Andhra Pradesh, India).
Acute Toxicity Study
Determination of LD50 : The acute toxicity [14] of alcoholic extract of roots of C. pareira was determined by using albino mice of either sex (16-20 g), maintained under standard husbandry conditions. The animals were fasted for 3h prior to the experiment and the extracts were administered as single dose and observed for the mortality up to 48h study period (short term toxicity). Based on the short term toxicity profile, the next dose of the extract was determined as per OECD guidelines No.420 upto the maximum dose level of 2000 mg/kg. From the LD50, dose of the extract, doses like 1/20th, 1/10th and 1/5th were selected and considered as low, medium and high dose i.e.: 100 mg/kg, 200 mg/kg, 400 mg/kg respectively to carry out this study.
Experimental Design
The antiurolithic activity of alcoholic extract of roots of C. pareira in albino rats was studied in Ammonium chloride (2% AC) and (0.75%) Ethylene glycol induced urolithiasis [15,16]. Healthy male albino rats weighing between 140-200 g were randomly divided into nine groups with each consisting of six animals and the treatment with AC, EG mixed water was continued for 10 days.
Group-1: Fed with standard rat chow diet and tap water only ad libitum for 10 days.
Group-2: Fed with normal rat diet + drinking water containing 0.75% EG v/v + 2% AC w/v for 10 days to induce urolithiasis.
Group-3: Fed with normal rat diet + drinking water containing 0.75% v/v EG + 2% w/v AC + Standard drug cystone (5 ml/kg) for 10 days.
Group-4: Fed with normal rat diet + alcoholic extract of roots of C. pareira lower dose (100 mg/kg) for 10 days.
Group-5: Fed with normal rat diet + alcoholic extract of roots of C. pareira medium dose (200 mg/kg) for 10 days
Group-6: Fed with normal rat diet + alcoholic extract of roots of C. pareira high doses (400 mg/kg) for 10 days.
Group-7: Fed with normal rat diet + drinking water containing 0.75 v/v EG + 2% w/v AC with alcoholic extract of roots of C. pareira lower dose (100 mg/kg) for 10 days.
Group-8: Fed with normal rat diet + drinking water containing 0.75 v/v EG + 2% w/v AC with alcoholic extract of roots of C. pareira medium dose (200 mg/kg) for 10 days
Group-9: Fed with normal rat diet + drinking water containing 0.75 v/v EG + 2% w/v AC with alcoholic extract of roots of C. pareira high dose (400 mg/kg) for 10 days.
Collection and Analysis of Urine
On 11th day three rats from each Group was kept in single metabolic cage and urine (pooled) collected for 24h. HCL was added to the urine before being stored at 4°C. Urine was measured for volume and analysed for biochemical parameters i.e.; calcium, magnesium and uric acid.
Serum Analysis
Blood was also collected on 11th day by retro orbital puncture under either anaesthesia and the animals were sacrificed by cervical decapitation. Serum was separated by centrifugation at 10, 000 rpm for 10 min and analysed for calcium, magnesium and creatinine.
Histopathological Studies
Kidneys collected from rats were weighed individually and fixed rapidly with 10% formalin. This slice section of kidneys fixed in paraffin were prepared and stained with eosin and hematoxylin and observed for histopathological changes.
Statistical Analysis
Experimental results were expressed as mean + SEM (n=6). Statistical analysis was performed with one way ANOVA followed by Dunnetts t-test.
Results
The alcoholic extract of roots of C. pareira was subjected to qualitative phytochemical tests to identify the phytoconstituents and the tests revealed the presence of carbohydrates, alkaloids, sterols, phenolic compounds, tannins, flavonoids and resins.
In acute toxicity study all the animals were survived even after 14 days indicates the non toxicity of the extract even up to the maximum permitted dose level of 2,000 mg/kg. No major behavioural changes were observed during this period of study.
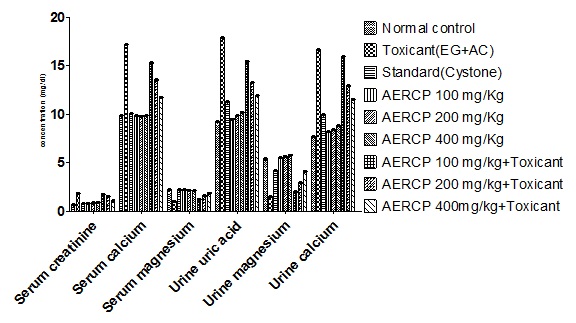
The results obtained with antiurolithic activity studies with alcoholic extract of roots of C. pareira were shown in [Table/Fig-1,2]. From the results when compared to normal control it can be observed that alcoholic extract of roots of C. pareira has shown a significant antiurolithic activity by increasing urinary output, magnesium and decreasing calcium, uric acid and decreasing serum creatinine, calcium and increasing magnesium levels. The antiurolithic effect observed after treatment with alcoholic extract of roots of C. pareira was found to be significant and comparable to standard cystone in terms of increase in urinary output and reduction in the tendency for crystallization.
Biochemical parameters noted with Groups of rats treated only with alcoholic extract of roots of C. Pareira (Per se effect) at different dose levels are compared with normal control and toxicant control. No significant difference in biochemical parameters observed confirming the non toxicity nature of these two extracts on the biological system of rats.
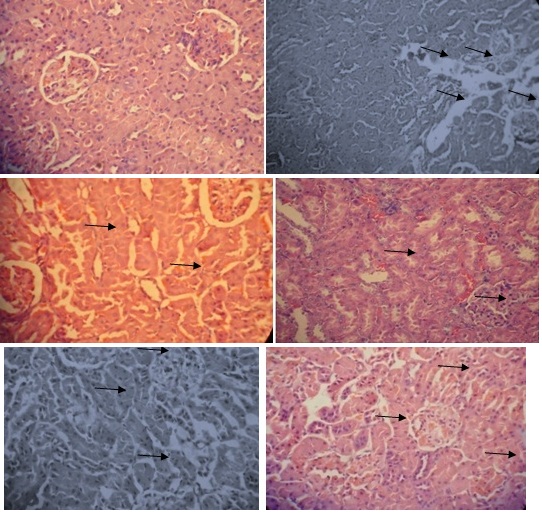
Histopathology of kidneys
In control Group (Group-I) histopathology of kidneys [Table/Fig-3] revealed no calcium oxalate deposits in the nephron segment. In urolithic rats (Group-II) several calcium oxalate crystal deposits inside the tubules and dilation of the proximal tubules along with interstitial inflammations and degeneration of epithelial cells were observed in the renal tissue. The Groups treated with alcoholic extract of roots of C. pareira (Groups VII –IX) and cystone treated rats (Group III) the number of calcium oxalate deposits in the tubules were less than Group II. In Groups treated with alcoholic extract of roots of C. pareira at 200 mg/kg and 400 mg/kg dose levels revealed less tissue damage and the cytology of the nephrotic tissue was observed near normal to Group I (normal) control rats.
Effect of AERCP on urine volume, urine calcium, urine uric acid, urine magnesium, serum calcium, serum creatinine, serum magnesium
| S. No | Group | Total Urine Volume | Serum Creatinine | Serum Calcium | Serum Magensium | Urine Uric Acid | Urine Magnesium | Urine Calcium |
| 1 | Normal Control | 16. ± 0.03 | 0.65±0.02 | 9.82±0.03 | 2.23±0.03 | 9.23±0.03 | 5.38±0.03 | 7.66±0.04 |
| 2 | Toxicant Control | 7.55 ± 0.03 | 1.85±0.01 | 17.14±0.04 | 0.98±0.04 | 17.84±0.02 | 1.51±0.02 | 16.60±0.03 |
| 3 | Standard | 15.20±0.04*** | 0.77±0.03*** | 10.04±0.04*** | 2.19±0.02*** | 11.24±0.03*** | 4.15±0.02*** | 9.90±0.04*** |
| 4 | AERCP 100 mg/Kg (Perse effect) | 18.94±0.01*** | 0.80±0.02*** | 9.82±0.01*** | 2.19±0.02*** | 9.47±0.02*** | 5.50±0.02*** | 8.16±0.02*** |
| 5 | AERCP 200 mg/Kg (Perse effect) | 21.36±0.02*** | 0.84±0.02*** | 9.80±0.02*** | 2.15±0.02*** | 9.86±0.02*** | 5.65±0.02*** | 8.40±0.02*** |
| 6 | AERCP 400 mg/Kg (Perse effect) | 25.12±0.02*** | 0.89±0.02*** | 9.82±0.02*** | 2.12±0.01*** | 10.18±0.02*** | 5.77±0.02*** | 8.83±0.02*** |
| 7 | AERCP 100 mg/Kg + Toxicant | 8.33 ± 0.02*** | 1.68±0.05ns | 15.26±0.02*** | 1.23±0.02*** | 15.43±0.03*** | 1.97±0.03*** | 15.91±0.04*** |
| 8 | AERCP 200 mg/Kg + Toxicant | 11.54±0.03*** | 1.49±0.06*** | 13.49±0.04*** | 1.56±0.03*** | 13.23±0.03*** | 2.89±0.03*** | 12.88±0.04*** |
| 9 | AERCP 400 mg/Kg + Toxicant | 14.40±0.03*** | 1.04±0.06*** | 11.73±0.03*** | 1.85±0.03*** | 11.89±0.03*** | 4.10±0.02*** | 11.50±0.04*** |
Values expressed as mean ± SEM, n=6, Significance at, and p<0.001***, ns = not-significant Compared with control Group (One-way ANOVA followed by Dunnetts `t’ test) , AERCP-Alcoholic extract of roots of C.pariera
Effect of CY Stone, AERCP and AERCP + Toxicant on urinary calcium, uric acid, magnesium, serum calcium, creatnine, magnesium (mg/dl) against ammonium chloride (AC 2%) and ethylene glycol (EG 0.75%) included urolithiasis
Histopathology of kidneys with AC (2%) and EG(0.75%) induced urolithiasis in albino rats
Discussion
In the present study, male rats were selected to induce urolithiasis because their urinary system resembles that of humans [17].
In view of its traditional use in renal calculi, C.pareira root extracts were studied to explore its potential as antiurolithic agent in (AC 2%) Ammonium chloride and (0.75%) Ethylene glycol induced urolithiasis. This is the first kind of the scientific work for the first time studied to show the antiurolithic effect of alcoholic extract of roots of C. pareira in urolithiasis model.
From the results it was observed that alcoholic extract of roots of C. pareira exhibited curative effect in urolithiasis induced rats by preventing the formation, reducing number and disruption of calcium oxalate calculi formed in the kidneys. Renal calcium oxalate deposition induced by ammonium chloride and ethylene glycol in rats is commonly used as a model to mimic the urinary calculi development in humans (Thamilselvan et al., Atmani et al., 2003; Tsai et al., 2008).Hence this model was used to evaluate the potential antiurolithic effect of alcoholic extract of roots of C. pareira on calcium oxalate urolithiasis.
In the present study alcoholic extract of roots of C. pareira treated animals showed an increase in urine output which dilutes the urinary electrolytes concentration. As a result, calcium and uric acid are flushed out via the urine leaving a lesser chance of precipitation with a decreased formation as well as the growth of urinary stone. The excretion of calcium and uric acid were gradually increased in stone induced animals which is in accordance with the earlier reports [18]. Nucleation and precipitation of calcium oxalate from urine and subsequent crystal growth [19] is favoured by increase in the urinary calcium. However, alcoholic extract of roots of C. pareira lowered the levels of calcium as well as uric acid, which is beneficial in preventing calculi formation.
Magnesium powerfully inhibits the crystallization of Calcium Oxalate invitro, magnesium binds to oxalate to form a soluble complex, subsequently reducing the concentration available for Calcium oxalate precipitation [20]. Low urinary magnesium content is a common feature in stone formers. Treatment with alcoholic extract of roots of C. pareira significantly increased the levels of magnesium in urine and serum but significantly reduced in ethylene glycol and ammonium chloride treated (Group-II) animals.
In the present work alcoholic extract of roots of C. pareira was studied for their antiurolithic activity.The phytochemical studies reveals that the roots of C. pareira contains flavonoids, alkaloids, carbohydrates, sterols, phenolic compounds, tannins, resins. From the earlier studies it has been reported that flavanoids [21,22] alkaloids, saponins [22] have antiurolithic activity. Earlier studies reported phytochemical substances like flavonoids, saponins, organic acids, steroids, carbohydrates, tannins, phenolic compounds, terpenoids, alkaloids, glycosides, sterols, sesquiterpenes and aminoacids, carotinoids in different plant extracts. Alcoholic extract of roots of C. pareira was identified with most of these plant phytochemical substances mentioned above. Hence, it can be reported that the observed antiurolithic activity is due to these above phytoconstituents. Phytoconstituent like berberine [4] is already reported for its antiurolithic activity. Berberine is an important bioactive constituent present in C. pareira. So here benzyl isoquinoline alkaloid berberine is responsible for antiurolithic activity because it was therapeutically effective for both prevention as well as curative aspect of calcium oxalate urolithiasis, exhibiting these effects through a combination of antioxidant, diuretic, hypocalciuric, hypermagnesiemia and urine alkalanizing activities.
Conclusion
Results showed that alcoholic extract of roots of C. pareira has exhibited a significant protective (antiurolithic) effect against urolithiasis producing agents. The rats treated with alcoholic extract of roots of C. pareira at doses 100 mg/kg, 200 mg/kg and 400 mg/ kg significantly (p≤ 0.05) reduced serum calcium and creatinine but increased magnesium. Further urinary calcium, uric acid levels were significantly decreased but urinary magnesium increased. In traditional medicine the plant is used for its antiurolithic activity. Ours scientific study come up with identification of so many phytochemical constituents reported earlier for this antiurolithic effect.Thus the present study supports and justify the basis for folklore use of roots of C. pareira for antiurolithic activity.
Acknowledgement
The authors are very much grateful to Dr. S.M. Shanth Kumar Ex Principal, and Dr. V. Hemanth Kumar Principal, VL College of Pharmacy, India. Raichur, Karnataka, India for providing the laboratory facilities to carry out the part of this Ph.D experimental work and Dr. Pramod Kumar, Pharmacognocist for authentication of roots of C. Pareira, Dr. Shashidhar Basagoudar, Assitant Professor, Department of Preventive & Social medicine (P&SM), RIMS, Raichur, Karnataka, India for helping in the preparation of this manuscript.
[1]. HG Tiselius, Aetiology and investigation of stone disease.Curriculum in urology. Eur J Urol. 1998 2(1):1-7. [Google Scholar]
[2]. C Ramesh, K Nandakumar, R Rajesh, Antiurolithic activity of heart wood extract of Cedrus deodara in ratsJ Compl Integr Med 2010 7(1):11 [Google Scholar]
[3]. SS Agrawal, BP Tamrakar, M Paridhavi, Clinically useful herbal drugs 2009 1st EditionNew DelhiAhuja publishers:76 [Google Scholar]
[4]. G Amaresh, PN Singh, CV Rao, Antinociceptive and antiarthritic activity of Cissampelos pareira roots.J Ethnopharmacol 2007 111:531-6. [Google Scholar]
[5]. BK Singh, K Kohli, SE Haque, Effect of Cissampelos pareira extract on isoproterenol induced cardiac dysfunctionJ Nat Med 2013 67(1):51-60. [Google Scholar]
[6]. T Issat, M Jakobisiak, Berberine Golab J, A natural cholesterol reducing product exerts anti tumor cytotoxic effects independently from the mevalonate pathway.Oncol Rep 2006 16(6):1273-6. [Google Scholar]
[7]. G Amresh, GD Reddy, CV Rao, PN Singh, Evaluation of anti-inflammatory activity of Cissampelos pareira roots in albino rats.J Ethnopharmacol 2007 110:526-31. [Google Scholar]
[8]. G Amresh, GD Reddy, CV Rao, Ethnomedical value of Cissampelos pareira extract in experimentally induced diarrhea.Acta Pharma 2004 54(1):27-35. [Google Scholar]
[9]. M Ganguly, M Borthakur, N Devi, R Mahanta, Antifertility activity of the methonolic leaf extract of Cissampelos pareira in female albino miceJ Ethnopharmacol 2007 111:688-91. [Google Scholar]
[10]. M Ye, S Fu, R Pi, F He, Neuropharmacological and pharmacokinetic properties of berberine: a review of recent researchJ Pharm Pharmacol 2009 61(7):831-7. [Google Scholar]
[11]. S Surendran, E Bhavani, M Vijaykumar, CH Rao V, In vitro and in vivo hepatoprotective activity of Cissampelos pareira aganist carbon tetra chloride induced hepatic damage.Ind J Exp Biol. 2011 49(12):939-45. [Google Scholar]
[12]. A Bafna, S Mishra, Antioxidant and immunomodulatory activity of the alkaloidal fraction of Cissampelos pareira L.Sci Pharm 2010 78(1):21-31. [Google Scholar]
[13]. S Amritpal, D Sanjiv, S Jaswinder, K Shankar, An inside preview of ethnopharmacology of Cissampelos pareira L.Int J Bio Tech 2010 1(1):114-20. [Google Scholar]
[14]. OECD guidelines on acute oral toxicity, Environmental health and safety monograph series on testing and adjustment 2001 425 [Google Scholar]
[15]. KM Suman, M Satyaranjan, S Sahoo, PK Panda, Antiurolithic activity of Crataeva magna Lour BarkInd J Nat Prod and resour 2011 2(1):28-33. [Google Scholar]
[16]. A Khan, S Khan, A Gilani, Studies on the in vitro and in vivo antiurolithic activity of Holarrhena antidysentericaUrol Res 2012 46:671-81. [Google Scholar]
[17]. TK Richardson, NE Tolbert, Oxidation of glycolic acid to oxalic acid by glycolic acid oxidase.J Biol Chem 1961 57:816-32. [Google Scholar]
[18]. K Divakar, AT Pawar, SB Chandrasekhar, SB Dighe, G Divakar, Protective effect of the hydro-alcoholic extract of Rubia cordifolia roots against ethylene glycol induced urolithiasis in rats.Food Chem Toxicol 2010 48:1013-18. [Google Scholar]
[19]. K Roger, MD Low, ML Stoller, Uric acid nephrolithiasisUrol Clin North Am 1997 24:135-48. [Google Scholar]
[20]. HG Rushton, M Spector, Effects of magnesium deficiency on intratubular calcium oxalate formation and crystalluria in hyperoxaluric ratsJ Urol 1982 127:598-604. [Google Scholar]
[21]. AK Rad, MAR Hadjzadeh, Z Rajael, N Mohammadian, S Valiollahi, The beneficial effect of Cynodon dactylon fractions on ethylene glycol induced kidney calculi in ratsUro J. 2011 8(3):179-84. [Google Scholar]
[22]. M Touhami, A Laroubi, K Elhabazi, F Loubna, I Zrara, Lemon juice has protective activity in a rat urolithiasis modelBMC Uro 2007 7(18) [Google Scholar]