Background: Interleukin-18 (IL-18) is a pro inflammatory cytokine which plays a key role in the acute and chronic inflammatory phases of Rheumatoid Arthritis (RA). The Single Nucleotide Polymorphisms (SNPs) of IL-18 gene promoter region at positions -137 and -607, are postulated to be associated with RA. To test this, this study aimed to identify the association between these SNPs of the IL-18 gene promoter region of RA in south Indian patients.
Materials and Methods: This study was carried on 190 subjects among which 90 were RA patients and 100 were age and sex matched controls. Genomic DNA was extracted by Salting out method. IL 18 gene promotor region SNPs, IL 18 - 607 and IL 18 -137 were amplified by using sequence specific primers. The amplified products of different samples were separated by using a 1.5% agarose gel, stained with ethidium bromide and photographed. All statistical analyses were carried out by using SYSTAT 12 software.
Results: At position 607, the frequencies of C allele, CC genotype, A allele and AA genotype were found to be significantly higher in patients and controls respectively and there was no significant difference in CA genotype. At position 137, there was no significant difference between the two groups with regard to G and C allelles but there was a significant increase in GG genotype of patients and CC genotype of controls. There was no association between duration of morning stiffness, rheumatoid factor positivity or negativity, age of onset and gender with distribution of genotypes and alleles.
Conclusion: C allele, CC genotype at position-607 and GG genotype at position-137 are risk factors and A allele, AA genotype at position-607 and CC genotype at position-137 have protective effect for RA.
Interleukin-18, Genotype, Pro inflammatory cytokine
Introduction
Rheumatoid Arthritis (RA) is a chronic, generally progressive, autoimmune disease that causes functional disability, significant pain and joint destruction [1]. In adult Indian population, a prevalence rate of 0.75% was observed [2]. This is a common disease seen in tropical areas [3]. Women are mostly affected by RA. It affects thrice as many women as men [4].
The pathogenesis of RA involves the following stages i.e. initiation, perpetuation and tissue damage. Each stage involves different cell and molecular interactions [5–6]. Interleukin-18 (IL-18) a pro inflammatory cytokine and it plays significant role in the pathogenesis of RA. The gene, IL-18 is mapped to short arm of chromosome 11 (11q22.2-22.3) [7]. IL-18 is found in the synovial tissues and enhanced levels of IL-18 are measured in the joints and sera of RA patients. IL-18 enhances the infiltration of inflammatory cells into the synovial tissues [8]. The role of IL-18 in RA is as follows; in chondrocytes, IL-18 increases gene expression for inducible nitric oxide synthetase, cyclooxygenase 2 and stromelysin [9]. Exposure of human articular cartilage to IL-18 increases the release of glycosaminoglycans, which are by-products of its degradation.
IL-18 protein expression is regulated by the IL-18 promoter gene [10,11] through two single nucleotide polymorphisms (SNPs), at positions -607 and -137 in the promoter region. These promoter regions are expected to be the binding sites for Cyclic (Adenosine 30, 50-cyclic monophosphate) AMP-responsive element-binding protein (CREB) [12] and Human histone H4 gene-specific transcription factor-1(H4TF-1) [13]. At position -607, the alteration from C-(cytosine) to A- (adenosine) nucleotide disrupts a potential CREB binding site and at position -137, the change from G- (guanine) to C- (cytosine) nucleotide affects the H4TF- 1-binding site. In the IL-18 gene promoter transcription activity assay, following stimulation, low promoter activities were observed for A and C alleles at positions -607 and -137, respectively. In contrast, higher promoter activities were observed for C and G alleles at similar positions [14]. RA patients may have higher IL-18 gene promoter transcription activities as causal factors of their disease pathogenesis. It has been postulated that RA patients would have higher frequencies of C alleles at position -607 and higher frequencies of G alleles at position -137 of the IL-18 promoter gene. Alternatively, higher frequencies of A allele at position -607 and higher frequencies of C allele at position -137 would confer protective effects against the development of RA. To test these postulations, this study aimed to identify the associations between these SNPs of the IL-18 promoter gene region in RA disease, in South Indian patients. In addition to its pro inflammatory activity in RA, IL-18 also contributes to other diseases such as Cancer [15], Crohn’s disease [16], Type 1 Diabetes [17,18] and Adult-Onset Still’s disease [19].
Materials and Methods
This study was carried out on 190 subjects, of which 90 (65 females and 25 males) were RA patients and 100 were age and sex matched controls. The laboratory work was done in Department of Human Genetics, Andhra University and Pediatric Research and Genetic Lab, Maulana Azad Medical College, New Delhi, India. All patients fulfilled the American College of Rheumatology (ACR) 1987 revised criteria for classification of RA [20] and had disease history of minimum three years. Five ml intravenous blood samples were collected from patients and controls into EDTA vacutainers under aseptic conditions after obtaining informed consents from subjects.
PCR Amplification
Genomic DNA was extracted by Salting out method [21] and it was quantified by using a spectrophotometer. An absorbance ratio of 1.8:2.0 or greater was considered and the final solution was stored at -40C. The set of sequence specific primers, as was illustrated in previous studies [22] was used to amplify the target DNA in the promoter region of IL-18 -137 and -607 and it has been summarized in [Table/Fig-1].
IL-18 promoter region gene primers (Sivalingam et al., 2003)
| IL18-607-F1 | 5’-GTTGCAGAAAGTGTAAAAATTATTAC-3’ |
| IL18-607-F2 | 5’-GTTGCAGAAAGTGTAAAAATTATTAA-3’ |
| IL18-607-R | 5’-TAACCTCATTCAGGACTTCC-3’ |
| IL18-607-CF | 5’-CTTTGCTATCATTCCAGGAA-3’ |
| IL18-137-F1 | 5’-CCCCAACTTTTACGGAAGAAAAG-3’ |
| IL18-137-F2 | 5’-CCCCAACTTTTACGGAAGAAAAC-3’ |
| IL18-137-R | 5’-AGGAGGGCAAAATG CACTGG-3’ |
| IL18-137-CF | 5’-CCAATAGGACTGATTAT TCCGCA-3’ |
Amplification of IL-18 -607
The Hot start period was performed for 2 minutes at 940C, followed by 940C - 20 seconds, 640C - 40 seconds, 720C - 40 seconds for 7 cycles and 940C - 20, seconds 570C - 40 seconds, 24 cycles, 720C - 40 seconds and 720C - 5 minutes.
IL-18 137 Amplification
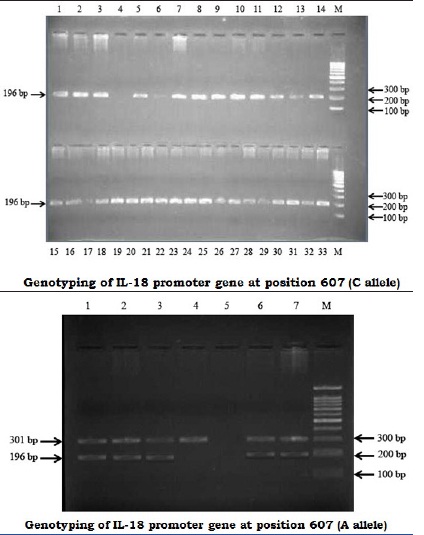
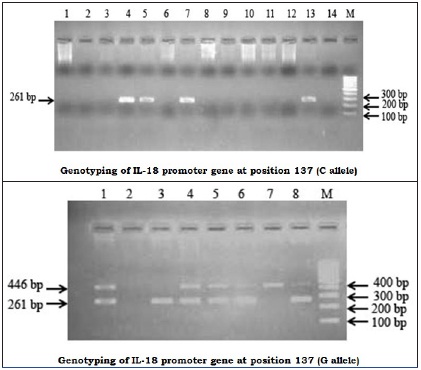
The Hot start period is for 2 minutes at 94°C, followed by 940C - 20 seconds, 680C - 60 seconds for 5 cycles and 720C - 40 seconds, 940C - 20 seconds, 620C - 40 seconds, 25 cycles and 720C - 40 seconds, 720C - 5 minutes. An amplification product of 196 bp was detected for position -607 and a 261 bp product was detected for position -137. The amplified products for different samples were separated by using a 1.5% agarose gel, stained with ethidium bromide and photographed.
Statistical Analysis
All statistical analyses were done by using SYSTAT 12 software. A two-sided t-test was used to compare continuous variables like Haemoglobin %, ESR and cell counts (total and differential) between rheumatoid factor (+ve) and rheumatoid factor (-ve) patients. The genotypic, allelic frequencies of subjects and the genotypic and allele frequencies with respect to gender, age of onset and clinical features (morning stiffness and rheumatoid factor) were compared by using z-test for comparison of proportions. In all the above statistical tests, a probability value of <0.05 was considered as statistically significant.
Results
The demographic and clinical factors have been summarized in [Table/Fig-2]. The female to male ratio was 2.6:1, mean disease duration was 5±5 years and the age of onset was 39±12 years. When the clinical profiles were compared between rheumatoid factor (+ve) and rheumatoid factor (-ve) patients, they showed no association between them [Table/Fig-3].
Demographic and Clinical factors of rheumatoid arthritis patients
| No | Parameters | Control | RA |
|---|
| 1 | Number of patients | 100 | 90 |
| 1.1 | Females | 50 | 65 |
| 1.2 | Males | 50 | 25 |
| 1.3 | Female to Male ratio (F:M) | 1:1 | 2.6:1 |
| 2 | Age (years) | 48±12 | 46 ± 12 |
| 3 | Age of onset (years) | NA | 39 ± 12 |
| 4 | Disease duration (years) | NA | 5 ± 5 |
| 5 | Rheumatoid Factor positive cases | NA | 47/87 (54.02%) |
| 5.1 | Females | NA | 34/64 (53.12%) |
| 5.2 | Males | NA | 13/23 (56.52%) |
| 6 | Erythrocyte sedimentation rate (ESR) | NA | 41 (6-110) |
| 7 | C-reactive protein (CRP) positive cases | NA | 24/87 (23%) |
| 7.1 | Females | NA | 16/64 (25%) |
| 7.2 | Males | NA | 5/23 (21.73%) |
Comparison of clinical profiles in sero positive and sero negative rheumatoid arthritis patients
| Parameter | RF+ve (Mean±SD) | RF-ve (Mean±SD) | p-value | Significance | Sd |
|---|
| HB (%) | 11 ± 2 | 11 ± 2 | 0.12 | NS | 2.96 |
| ESR (cm/hr) | 39 ± 18 | 44 ± 23 | 0.20 | NS | 2.2 |
| Total count | | 9004 ± 1921 | 0.75 | NS | 2.96 |
| Neutrophils | 65 ± 8 | 66 ± 7 | 0.78 | NS |
| Differential count | Lymphocytes | 29 ± 8 | 29 ± 7 | 0.93 | NS |
| Eosinophils | 4 ± 2 | 3 ± 2 | 0.26 | NS |
| Monocytes | 2 ± 1 | 2 ± 1 | 0.30 | NS |
RF+ve= rheumatoid factor positive, RF-ve= rheumatoid factor negative, p= p-value –probability value of the statistical test, SD= standard deviation, S= significant, NS= not significant
The alleles and genotype frequencies of IL 18 -607 have been presented in [Table/Fig-4,5]. At position 607, the frequencies of C allele, CC genotype, A allele and AA genotype were found to be significantly higher in patients and controls respectively. The difference was statistically significant (p=0.0000) and no significant difference was seen in CA genotype.
Allelic frequencies of IL-18 promoter gene at position -607 in rheumatoid arthritis patients and controls
| Alleles | RA Patients | Controls | p-value | Significance |
|---|
| n (160) | Frequency | n (200) | Frequency |
|---|
| A | 41 | 0.26 | 128 | 0.64 | 0.000 | S |
| C | 119 | 0.74 | 72 | 0.36 | 0.000 | S |
n = number of individuals, p-value – probability value of the statistical test, S = significant, NS= not significant
Genotypic frequencies of IL-18 promoter gene at position -607 in rheumatoid arthritis patients and controls
| Genotypes | RA Patients | Controls | p-value | Significance |
|---|
| n (80) | Frequency | n (100) | Frequency |
|---|
| CC | 42 | 0.52 | 10 | 0.10 | 0.000 | S |
| CA | 35 | 0.44 | 52 | 0.52 | 0.271 | NS |
| AA | 3 | 0.04 | 38 | 0.38 | 0.000 | S |
n – number of individuals; p-value – probability value of the statistical test, S= significant, NS= not significant
The allelic and genotypic frequencies of IL 18 -137 have been presented in [Table/Fig-6,7]. At position 137, no significant difference was seen between the two groups with regard to G and C allelles, but significant increases were observed in GG genotype of patients and CC genotype of controls (p=0.0000).
Allelic frequencies of IL-18 promoter gene at position -137 in rheumatoid arthritis patients and controls
| Alleles | RA Patients | Controls | p-value | Significance |
|---|
| n (140) | Frequency | n (200) | Frequency |
|---|
| G | 103 | 0.74 | 141 | 0.70 | 0.536 | NS |
| C | 37 | 0.26 | 59 | 0.30 | 0.536 | NS |
n = number of alleles, p-value = probability value of the statistical test, S= significant, NS= not significant
Genotypic frequencies of IL-18 promoter gene at position -137 in rheumatoid arthritis patients and controls
| Genotypes | RA Patients | Controls | p-value | Significance |
|---|
| n (70) | Frequency | n (100) | Frequency |
|---|
| GG | 42 | 0.60 | 20 | 0.20 | 0.000 | S |
| GC | 19 | 0.27 | 19 | 0.19 | 0.209 | NS |
| CC | 9 | 0.13 | 61 | 0.61 | 0.000 | S |
n – number of individuals; p-value – probability value of the statistical test, S= significant, NS= not significant
The distributions of genotypic and allelic frequencies of IL 18-607 and IL 18-137 in relation to duration of morning stiffness, rheumatoid factor (+ve) and rheumatoid factor (-ve), age of onset and gender have been given in [Table/Fig-8,9]. No significant association was seen between these parameters and distributions of genotypes and alleles of IL 18-607 and IL 18-137 polymorphism in RA [Table/Fig-10,11].
Genotyping of IL-18 promoter gene at position -607. A 1.5% agarose gel stained with ethidium bromide after PCR amplification for the position -607 genotyping. Amplification products of 196-bp for the alleles C and A (arrow) were detected. The 301-bp band corresponds to the PCR internal control. The right lane contains a 100-bp marker (M)
Genotyping of IL-18 promoter gene at position -137: A 2% agarose gel stained with ethidium bromide after PCR amplification for the position -137 genotyping. Amplification products of 261-bp for the alleles G and C were detected. The 446-bp band corresponds to the PCR internal control. The right lane contains a 100-bp marker (M)
Genotype and allele frequencies of IL 18-607 polymorphism in rheumatoid arthritis patients in relation to clinical features
| Geno type / allele | Morning Stiffness | Rheumatoid Factor | Age of onset | Gender |
|---|
| <1 (n=46) | >1 (n=18) | p-value | Significance | Positive (n=34) | Negative (n=30) | p-value | Significance | >35 years (n=47) | <35 years (n=25) | p-value | Significance | Females (n=46) | Males (n=19) | p-value | Significance |
|---|
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % |
|---|
| AA | 1 | 2.17 | 1 | 5.55 | 0.484 | NS | 1 | 2.94 | 1 | 3.33 | 0.928 | NS | 6 | 12.76 | 4 | 16.0 | 0.705 | NS | 2 | 4.34 | 1 | 5.26 | 0.872 | NS |
| CA | 22 | 47.82 | 8 | 44.44 | 0.807 | NS | 13 | 38.23 | 17 | 56.66 | 0.140 | NS | 23 | 48.93 | 7 | 28.0 | 0.086 | NS | 22 | 47.28 | 8 | 42.10 | 0.673 | NS |
| CC | 23 | 50.0 | 9 | 50.0 | 1.000 | NS | 20 | 58.82 | 12 | 40.0 | 0.132 | NS | 18 | 38.29 | 14 | 56.0 | 0.150 | NS | 22 | 47.28 | 10 | 52.63 | 0.724 | NS |
| A-allele | 24 | 26.08 | 10 | 27.77 | 0.845 | NS | 15 | 22.05 | 19 | 31.66 | 0.219 | NS | 35 | 37.23 | 15 | 30.0 | 0.385 | NS | 26 | 28.62 | 10 | 26.13 | 0.821 | NS |
| C-allele | 68 | 73.91 | 26 | 72.22 | 0.845 | NS | 53 | 77.94 | 41 | 68.33 | 0.219 | NS | 59 | 62.76 | 35 | 70.0 | 0.385 | NS | 66 | 71.73 | 28 | 73.68 | 0.821 | NS |
n – number of patients, <1 =less than one hour, >1 = more than one hour, <35 = less than 35 years, >35 =more than 35 years, p-value = probability value of the statistical test, S = significant, NS = not significant
Genotype and allele frequencies of il 18-137 polymorphism in rheumatoid arthritis patients in relation to clinical features
| Geno type / allele | Morning Stiffness | Rheumatoid Factor | Age of onset | Gender |
|---|
| <1 (n=45) | >1 (n=18) | P-value | Significance | Positive (n=34) | Negative (n=29) | P-value | Significance | >35 years (n=42) | <35 years (n=24) | P-value | Significance | Females (n=45) | Males (n=23) | P-value | Significance |
|---|
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % |
|---|
| GG | 27 | 60.0 | 11 | 61.11 | 0.935 | NS | 20 | 58.82 | 18 | 62.06 | 0.792 | NS | 22 | 52.38 | 16 | 67.66 | 0.258 | NS | 27 | 60.0 | 11 | 47.82 | 0.338 | NS |
| GC | 12 | 26.66 | 5 | 27.77 | 0.928 | NS | 10 | 29.41 | 7 | 24.13 | 0.638 | NS | 12 | 28.57 | 5 | 20.83 | 0.489 | NS | 10 | 22.22 | 7 | 30.43 | 0.459 | NS |
| CC | 6 | 13.33 | 2 | 11.11 | 0.810 | NS | 4 | 11.46 | 4 | 13.79 | 0.809 | NS | 8 | 19.04 | 3 | 12.5 | 0.492 | NS | 8 | 17.77 | 5 | 21.73 | 0.694 | NS |
| G-allele | 66 | 73.33 | 27 | 75.0 | 0.847 | NS | 50 | 73.52 | 43 | 74.13 | 0.938 | NS | 56 | 66.66 | 37 | 77.08 | 0.207 | NS | 64 | 71.11 | 29 | 63.04 | 0.338 | NS |
| C-allele | 24 | 26.66 | 9 | 25.0 | 0.847 | NS | 18 | 26.47 | 15 | 25.86 | 0.938 | NS | 28 | 33.33 | 11 | 22.91 | 0.207 | NS | 26 | 28.88 | 17 | 36.95 | 0.338 | NS |
n = number of patients, <1 =less than one hour, >1 = more than one hour, <35 = less than 35 years, >35 =more than 35 years, p value = probability value of the statistical test, S = significant, NS = not significant
Discussion
The studies done on IL-18 in RA are conflicting and inconsistent. An earlier report made by Sivalingam et al., [22] on RA patients studied in Singapore showed similar results for position -607 i.e. high frequencies of A and C alleles among controls and patients respectively, but they were statistically insignificant. Regarding genotypic frequencies, genotype AA was found to be significantly higher in controls and no statistical significance was found for CA and CC genotypes. The allelic or genotypic frequencies seen at position -137 between subjects were also insignificant. Rueda et al., [23] and Pawlik et al., [24] reported that, IL-18-137 and -607 promoter polymorphisms were not significant with respect to RA courses and severities. Pan et al., [25] reported that IL-18 gene promoter -607 A/C polymorphism was not associated with development of autoimmune diseases. Ying et al., [26] reported that the genotype and allele frequency of IL-18-607 were not associated with IL-18 serum levels. Huang et al., [27] reported that IL-18-607 polymorphism was associated with RA, but not with IL-18-137 polymorphisms. Gracie et al., [28] reported that both SNPs found at positions -137 and -607 were involved in pathogenesis of RA. When other auto immune diseases were considered, the results were found to be very conflicting. Takada et al., [29] reported that C allele, at position -607, was a risk factor for sarcoidosis in the Japanese population. Zhou et al., [30] reported that it was unlikely for these SNPs to confer susceptibility to sarcoidosis [Table/Fig-10,11].
Conclusion
On comparing results of the above studies and our findings, it was concluded that C allele and CC genotype at position -607 and GG genotype at position-137 were risk factors for RA. A allele and AA genotype at position-607 and CC genotype at position -137 had protective effects.
Despite these encouraging findings, our study had few limitations. It did not include patients with intermediate and mild severities and also, the number of subjects which was studied was small. By resolving these limitations, this study can be made useful, because the polymorphisms which have been studied here have been linked with progression of the disease. These polymorphisms, therefore, provide a simple, rapid and cost effective tool for prediction of RA. Further, because these polymorphisms do not change over time, they can be used at an early stage of the disease course, in order to individualize therapy and to minimize articular damage.
RF+ve= rheumatoid factor positive, RF-ve= rheumatoid factor negative, p= p-value –probability value of the statistical test, SD= standard deviation, S= significant, NS= not significant
n = number of individuals, p-value – probability value of the statistical test, S = significant, NS= not significant
n – number of individuals; p-value – probability value of the statistical test, S= significant, NS= not significant
n = number of alleles, p-value = probability value of the statistical test, S= significant, NS= not significant
n – number of individuals; p-value – probability value of the statistical test, S= significant, NS= not significant
n – number of patients, <1 =less than one hour, >1 = more than one hour, <35 = less than 35 years, >35 =more than 35 years, p-value = probability value of the statistical test, S = significant, NS = not significant
n = number of patients, <1 =less than one hour, >1 = more than one hour, <35 = less than 35 years, >35 =more than 35 years, p value = probability value of the statistical test, S = significant, NS = not significant
[1]. Kvien TK, Epidemiology and burden of illness of rheumatoid arthritisPharmaco economics 2004 22(2 Suppl 1):1-12. [Google Scholar]
[2]. Malaviya AN, Kapoor SK, Singh RR, Kumar A, Pande I, Prevalence of rheumatoid arthritis in the adult Indian populationRheumatol Int 1993 13(4):131-34. [Google Scholar]
[3]. Diouf ML, Diallo S, Mbengue M, Moreira-Diop T, Methotrexate, liver and rheumatoid arthritis in tropical areasSante 2001 11(3):195-200. [Google Scholar]
[4]. Danneskiold-Samsoe B, Bartels EM, Dreyer L, Gender differences in autoimmune diseases illustrated by rheumatoid arthritisUgeskr Laeger 2007 169(25):2440-2. [Google Scholar]
[5]. Feldmann M, Brennan FM, Williams RO, Cope AP, Gibbons DL, Katsikis PD, Evaluation of the role of cytokines in autoimmune disease: the importance of TNF alpha in rheumatoid arthritisProg Growth Factor Res 1992 4(3):247-55. [Google Scholar]
[6]. Magalhaes R, Stiehl P, Morawietz L, Berek C, Krenn V, Morphological and molecular pathology of the B cell response in synovitis of rheumatoid arthritisVirchows Arch 2002 441(5):415-27. [Google Scholar]
[7]. Thompson SR, Humphries SE, Interleukin-18 genetics and inflammatory disease susceptibilityGenes Immun 2007 Mar 8(2):91-99. [Google Scholar]
[8]. Leung BP, McInnes IB, Esfandiari E, Wei XQ, Liew FY, Interleukin-18 can promote synovial inflammation through activation of peripheral blood and synovial neutrophilsArthritis Rheum 2000 43:1253 [Google Scholar]
[9]. Olee T, Hashimoto S, Quach J, Lotz M, IL-18 is produced by articular chondrocytes and induces pro inflammatory and catabolic responsesJ Immunol 1999 162(2):1096-100. [Google Scholar]
[10]. Tone M, Thompson SA, Tone Y, Fairchild PJ, Waldmann H, Regulation of IL-18 (IFN gamma-inducing factor) gene expressionJ Immunol 1997 159:6156-63. [Google Scholar]
[11]. Marshall JD, Aste-Amezaga M, Chehimi SS, Murphy M, Olsen H, Trinchieri G, Regulation of human IL-18 mRNA expressionClin Immunol 1999 90:15-21. [Google Scholar]
[12]. Haus-Seuffert P, Meisterernst M, Mechanisms of transcriptional activation of cAMPresponsive element-binding protein CREBMol Cell Biochem 2000 212:5-9. [Google Scholar]
[13]. Dailey L, Roberts SB, Heintz N, Purification of the human histone H4 gene-specific transcription factors H4TF-1 and H4TF-2Genes Dev 1988 2:1700-12. [Google Scholar]
[14]. Giedraitis V, He B, Huang WX, Hillert J, Cloning and mutation analysis of the human IL-18 promoter: a possible role of polymorphisms in expression regulationJ Neuroimmunol 2001 112:146-52. [Google Scholar]
[15]. Pages F, Berger A, Lebel-Binay S, Proinflammatory and antitumor properties of interleukin-18 in the gastrointestinal tractImmunol Lett 2000 75(1):9-14. [Google Scholar]
[16]. Pizarro TT, Michie MH, Bentz M, Woraratanadharm J, Smith MF Jr, Foley E, IL-18, a novel immunoregulatory cytokine, is up-regulated in Crohn’s disease: expression and localization in intestinal mucosal cellsJ Immunol 1999 162(11):6829-35. [Google Scholar]
[17]. Dong GP, Yu ZS, Liang L, Zou CC, Fu JF, Wang CL, IL-18 gene promoter-137C/G and -607C/A polymorphisms in Chinese Han children with type 1 diabetes mellitusInt J Immunogenet 2007 34(2):75-9. [Google Scholar]
[18]. Mojtahedi Z, Naeimi S, Farjadian S, Omrani GR, Ghaderi A, Association of IL-18 promoter polymorphisms with predisposition to Type 1 diabetesDiabet Med 2006 23(3):235-9. [Google Scholar]
[19]. Sugiura T, Kawaguchi Y, Harigai M, Terajima-Ichida H, Kitamura Y, Furuya T, Association between adult-onset Still’s disease and interleukin-18 gene polymorphismsGenes Immun 2002 3(7):394-9. [Google Scholar]
[20]. Arnett FC, Edworthy SM, Bloch DA, Mcshane DJ, Fries JF, Cooper NS, American rheumatism association 1987 revised criteria for the classification of rheumatoid arthritisArthritis Rheum 1998 31(3):315-24.From the Rheumatoid Arthritis Criteria Subcommittee of the Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association [Google Scholar]
[21]. Miller SA, Dykes DD, Polesky HF, A simple salting out procedure for extracting DNA from human nucleated cellsNucleic Acids Res 1988 16(3):1215 [Google Scholar]
[22]. Sivalingam SP, Yoon KH, Koh DR, Fong KY, Single-nucleotide polymorphisms of the interleukin-18 gene promoter region in rheumatoid arthritis patients: protective effect of AA genotypeTissue Antigens 2003 62:498-504. [Google Scholar]
[23]. Rueda B, Gonzalez-Gay MA, Mataran L, Lopez-Nevot MA, Martin J, Interleukin-18-Promoter polymorphisms are not relevant in rheumatoid arthritisTissue Antigens 2005 65(6):544-48.Rueda, et al., (2005) [32] [Google Scholar]
[24]. Pawlik A, Kurzawski M, Czerny B, Gawronska-Szklarz B, Drozdzik M, Herczynska M, Interleukin-18 promoter polymorphism in patients with rheumatoid arthritisTissue Antigens 2006 67(5):415-18.Pawlik, et al., (2006)[33] [Google Scholar]
[25]. Pan HF, Leng RX, Ye DQ, Lack of association of interleukin-18 gene promoter -607 A/C polymorphism with susceptibility to autoimmune diseases: a meta-analysisLupus 2011 20(9):945-51.Pan et al., [Google Scholar]
[26]. Ying B, Shi Y, Pan X, Song X, Huang Z, Niu Q, Association of polymorphisms in the human IL-10 and IL-18 genes with rheumatoid arthritisMol Biol Rep 2011 38(1):379-85. [Google Scholar]
[27]. Huang XZ, Zhuang JH, Ren YG, Zhou LJ, Zhou Q, Association of interleukin-6 and interleukin-18 gene polymorphism with rheumatoid arthritis in Guangdong Han populationNan Fang Yi Ke Da Xue Xue Bao 2007 27(11):1661-64. [Google Scholar]
[28]. Gracie JA, Koyama N, Murdoch J, Field M, McGarry F, Crilly A, Disease association of two distinct interleukin-18 promoter polymorphisms in Caucasian rheumatoid arthritis patientsGenes Immun 2005 6(3):211-16. [Google Scholar]
[29]. Takada T, Suzuki E, Morohashi K, Gejyo F, Association of single nucleotide polymorphisms in the IL-18 gene with sarcoidosis in a Japanese populationTissue Antigens 2002 60(1):36-42. [Google Scholar]
[30]. Zhou Y, Yamaguchi E, Hizawa N, Nishimura M, Sarcoidosis. Roles of functional polymorphisms in the interleukin-18 gene promoter in sarcoidosisVasc Diffuse Lung Dis 2005 22(2):105-13. [Google Scholar]