Non-Pigmented Strain of Serratia Marcescens: An Unusual Pathogen Causing Pulmonary Infection in A Patient with Malignancy
Priyamvada Roy1, Nishat Hussain Ahmed2, R.K Grover3
1Senior Resident, Department of Microbiology, Delhi State Cancer Institute, India.
2Assistant Professor, Department of Laboratory Medicine, Delhi State Cancer Institute, India.
3Director and Chief Executive Officer, Delhi State Cancer Institute, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Priyamvada Roy, Care of Group Captain D D Roy, Flat Number C-115, Jalvayu Vihar (Near AWHO), Plot Number 8, Pocket P-4, Builder’s Area, Greater Noida, Uttar Pradesh, Pin Code-201310
Phone: 9953820283,
E-mail: priyamvadaroy@yahoo.in
Serratia marcescens is a member of the family Enterobacteriaceae. It has emerged in recent years as an opportunistic pathogen of nosocomial infections. Some biotypes of Serratia marcescens produce the non-diffusible red pigment prodigiosin. Though both pigmented and non-pigmented biotypes may be pathogenic for humans, the non-pigmented biotypes are more virulent due to cytotoxin production and presence of plasmids mediating antibiotic resistance. However in India only one study done 31 years back has reported on infections caused by non-pigmented strains of Serratia marcescens. We present a case of a patient with squamous cell carcinoma of the left retromolar trigone, soft palate and buccal mucosa, who developed pulmonary infection with non-pigmented strain of Serratia marcescens. According to the available literature, this is the second report on infection with non-pigmented strain of Serratia marcescens from India. It is imperative to accurately detect the non-pigmented biotypes due to their tendency to cause serious and difficult to treat infections.
Drug-resistance, Enterobacteriaceae, nosocomial infections, squamous cell carcinoma, VITEKR 2 Compact
Case Report
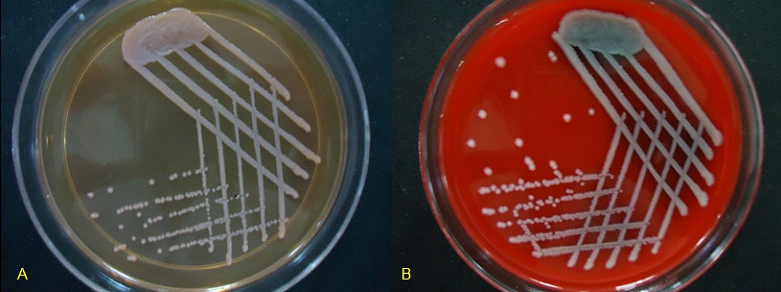
A 64 year old male patient having squamous cell carcinoma of the left retromolar trigone, soft palate and buccal mucosa, undergoing chemotherapy with paclitaxel, carboplatin and ifosfamide at Delhi State Cancer Institute for eight months, presented with fever, cough with expectoration and breathlessness on 20th November, 2013. His hematocrit parameters showed leucopenia (3510/μl) with monocytosis (Neutrophils-58%, Lymphocytes-20%, Monocytes-21%, Eosinophils-1%, Basophils-0%). Good quality sputum sample was collected aseptically and processed by standard microbiological techniques. Gram stain of the sputum sample revealed leukocytes and gram-negative bacilli. The sample was cultured on Blood agar and MacConkey agar [Table/Fig-1]. After overnight incubation at 37ºC on Blood agar, large colonies; 2-3 mm in diameter, convex, non-hemolytic, mucoid in consistency and non-pigmented were obtained. On MacConkey agar non-lactose fermenting colonies with similar morphology were seen. Gram stain of the growth showed gram negative, non-sporing and non-capsulated bacilli. The bacteria were motile in nature. The colonies were subjected to automated VITEKR 2 Compact(C) (Biomeriux, North Carolina / USA) Identification with gram negative GN REF 21341 identification (GNID) card. The isolate was identified as Serratia marcescens. The pathogenicity of this bacterium was confirmed by promptly culturing a repeat sample from the patient. Antibiotic susceptibility of the isolate was done in VITEK with AST Card N280. Antibiogram results were expressed as susceptible, intermediate or resistant according to the criteria of the Clinical Laboratory Standards Institute (CLSI) M100- S23 (2013) [1]. The bacterium was found to be sensitive to amikacin (minimum inhibitory concentration or MIC≤2μg/ml), gentamicin (MIC≤1μg/ml), piperacillin/tazobactam (MIC≤4μg/ml), cefepime (MIC≤1μg/ml), cefotaxime (MIC≤1μg/ml), ceftriaxone (MIC≤1μg/ml), ciprofloxacin (MIC≤0.25μg/ml), imipenem (MIC=0.5μg/ml), meropenem (MIC≤0.25μg/ml), ertapenem (MIC≤0.5μg/ml) and trimethoprim-sulfamethoxazole (MIC≤20μg/ml), and resistant to amoxicillin/clavulanic acid (MIC≥32μg/ml), cefuroxime (MIC≥64μg/ml) and cefuroxime axetil (MIC≥64μg/ml). The patient was successfully treated with intravenous amikacin and cefotaxime. He was also started on injection Granulocyte colony stimulating factor (G-CSF) to reverse the myelosuppression induced by cytotoxic drugs. The leucocyte count raised and came back to normal (7700/ μl) within a fortnight.
Growth of non-pigmented strain of . obtained after 24 hours of incubation at 37˚C on MacConkey Agar (A), and Blood Agar (B). (A) – On MacConkey agar colonies are 2-3 mm in diameter, convex, non-lactose fermenting, mucoid and non-pigmented. (B)- On Blood agar colonies are 2-3 mm in diameter, convex, non-hemolytic, mucoid and non-pigmented.
Report of Infections in India Caused by Serratia marcescens
| Serial No. | Study By | Year of Publication | Source of Isolate(s) | Pigmentation |
| 1. | Bhujwala et al[3] | 1983 | Urine, purulent discharge | 56% of isolates pigmented, 44% of isolates non-pigmented |
| 2. | Rastogi et al[4] | 2002 | Sputum | Pigmented |
| 3. | Dhawan et al [5] | 2003 | Pus | Pigmented |
| 4. | Sharma et al [6] | 2006 | Pleural fluid | Pigmented |
| 5. | Harapriya et al [7] | 2013 | Sputum | Pigmented |
| 6. | Khanna et al [8] | 2013 | Blood, urine, pus | Pigmented |
| 7. | Deodhar et al [9] | 1984 | Pus, sputum, blood, bronchoscopic aspirate | Pigmented |
Discussion
Serratia marcescens is an opportunistic, gram negative,nosocomial pathogen which belongs to the family Enterobacteriaceae. At present, Serratia marcescens is the only known nosocomial species of Serratia [2]. From India, there have been several reports on infections caused by pigmented strains of Serratia marcescens [3-9], but infections caused by the non-pigmented strains of has been reported only once in a study done at All India Institute of Medical Sciences 31 years ago [3]. [Table/Fig-2] gives an exhaustive list of published cases of Serratia marcescens infections in India till date. Based on available literature and to the best of our knowledge, we present the second report on infections due to non-pigmented strain of Serratia marcescens from India and the first report of the organism causing pulmonary infection in a patient with squamous cell carcinoma of the left retromolar trigone, soft palate and buccal mucosa in India.
Until the late 1950s, Serratia spp. were rarely isolated from human patients. Later on Serratia marcescens became more and more frequently involved in nosocomial infections and non-pigmented Serratia marcescens strains are now a serious threat in surgical and intensive care units [2].As Serratia marcescens is a normal commensal of alimentary canal [4], the sample was repeated in the present case to rule out the possibility of contamination. Isolation of the same organism in pure culture indicated its role in causation of the disease.
Some species and biotypes of Serratia produce a non-diffusible red pigment, prodigiosin, or 2-methyl-3-amyl-6-methoxyprodigiosene. However, several bacterial species outside the genus Serratia produce prodigiosin or prodigiosin-like pigments or many other kinds of red pigments, and the identity of microorganisms involved in these striking phenomena can only be surmised. In the genus Serratia, prodigiosin is only produced by strains of Serratia marcescens, Serratia plymuthica, and Serratia rubidaea. Pigmented biotypes of Serratia marcescens are mostly recovered from natural environments, whereas the non-pigmented biotypes are prevalent in the hospital. In Serratia marcescens, prodigiosin is produced by biogroups A1 and A2/6 and never by biogroups A3, A4 or A5/8. Non-pigmented strains of biogroups A1 or A2/6 are often blocked in the synthesis of either 2-methyl-3-amylpyrrole or 4-methoxy-2,2’- bipyrrole-5-carboxyaldehyde, which are essential components of the biosynthetic pathway of prodigiosin. Strains in the nonpigmented biogroups are likely to lack the condensing enzyme [2]. The isolate in the present case may belong to one of these biogroups. Serratia marcescens is an important cause of nosocomial infections and non-pigmented strains are more frequent than pigmented ones among clinical isolates [10].
Non-pigmented strains of Serratia marcescens are generally more resistant to antibiotics than pigmented strains because they often harbour resistance plasmids [2]. Infections caused by the organism may be difficult to treat because of resistance to a variety of antibiotics, including ampicillin and first and second generation cephalosporins [11] as in the present case. The antibiotics most often active against nosocomial strains of Serratia marcescens are amikacin, moxalactam, and cefotaxime [2]. The patient in the present case responded well to amikacin and cefotaxime. Recently, cytotoxin production was detected in non-pigmented isolates of Serratia marcescens, and this characteristic has been considered an important virulence factor in several species of bacteria. However, the occurrence of cytotoxin in pigmented Serratia marcescens remains to be demonstrated [10]. Therefore due to the presence of drug-resistant plasmids and cytotoxin production, the nonpigmented strains of 3 have emerged as significant pathogens of nosocomial infections.
Conclusion
Serratia marcescens is an uncommon opportunistic organism which can cause a wide variety of infections if ignored. Non-pigmented strains of Serratia marcescens are more pernicious due to drugresistance and cytotoxin production. However, due to the lack of the characteristic red pigment produced by most strains of Serratia marcescens, the non-pigmented strains may be missed and misidentified as other members of the family Enterobacteriaceae. Therefore we strongly recommend greater prudence towards proper identification of this organism as though this bacterium was earlier considered innocuous, it is now gaining momentum in causing human infections and the non-pigmented biotypes are more threatening due to antibiotic resistance and cytotoxin production.
[1]. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Twenty first Informational supplementCLSI document 2013 :M100-S23. [Google Scholar]
[2]. F Grimont, PAD Grimont, The Genus Serratia. In: Dworkin M, Falkow S, Rosenberg E, Schleifer KH, Stackebrandt E,Springer 2006 3rd EditionNew YorkProkaryotes.:219-244. [Google Scholar]
[3]. RA Bhujwala, Sriniwas S Dutta, Epidemiological study of Serratia marcescens infection in a hospital.Indian J Med Res 1983 78:29-36. [Google Scholar]
[4]. V Rastogi, P Purohit, BP Peters, PS Nirwan, Pulmonary Infection with Serratia marcescens.Indian J Med Microbiol 2002 20(3):167-168. [Google Scholar]
[5]. B Dhawan, R Bonnet, NK Shukla, P Mathur, BK Das, A Kapil, Infection with an Extended-Spectrum β-Lactamase Producing Strain of Serratia marcescens following Tongue Reconstruction.J Clin Microbiol 2003 41(5):2233-2234. [Google Scholar]
[6]. R Sharma, B Sharma, P Sinha, S Rishi, Empyema Thoracis Caused By Serratia marcescens in a 2-year Old ChildIndian J Med Sci. 2006 60(9):387-388. [Google Scholar]
[7]. K Harapriya, S Revati, D Bhaskar, S Sharvari, H Anahita, AD Urhekar, Pulmonary Infection with Serratia marcescens in a Tertiary Care Centre in Navi Mumbai, India.International Journal of Pharmaceutical Research and Bioscience 2013 2(4):227-229. [Google Scholar]
[8]. A Khanna, M Khanna, A Aggarwal, Serratia marcescens-A Rare Opportunistic Nosocomial Pathogen and Measures to Limit its Spread in Hospitalized Patients.J Clin Diagn Res 2013 7(2):243-246. [Google Scholar]
[9]. LP Deodhar, UM Tendolkar, Nosocomial infections due to Serratia marcescensJ Postgrad Med. 1984 30:89-90. [Google Scholar]
[10]. GV Carbonell, HHM Della Colleta, T Yano, ALC Darini, CV Levy, BAL Fonseca, Clinical Relevance and Virulence Factors of Serratia marcescensFEMS Immuno Med Microbiol 2000 28:143-149. [Google Scholar]
[11]. A Hejazi, FR Falkiner, Serratia marcescensJ Med Microbiol 1997 46:903-912. [Google Scholar]