Gram negative bacteria are known to become multidrug resistant, especially to betalactam antibiotics, and the most common mechanism of bacterial resistance to β-lactam is the production of β-lactamases such extended spectrum beta lactamases and metallo-β-lactamases [1]. BL+BLI (betalactamase and betalactamase inhibitor ally) are used for ESBL producing isolates, but carbapenems seemed to be the best options to treat patients with ESBL. Unfortunately, emergence of metallo-beta-lactamases restricted the use of carbapenems [2]. In India, the prevalence of MBLs range from 8% to 79% [3–6]. Moreover these isolates are resistant to other group of antibiotics as well. Looking beyond Carbapenems, options are very narrow, with colistin in focus and rather less hopeful results with Tigecycline. [7,8] It’s high time to look ahead for newer possibilities. However new antibiotic discovery and development has not kept pace with the existing demand of the same.
An alternate perspective is use of potentiators of the already existing antibiotics the so called antibiotic adjuvants. Antibiotic adjuvants are active molecules, preferably with non-antibiotic activity, that in combination with antibiotics, enhance the antimicrobial activity of the latter. These compounds can function either by reversing resistance mechanisms in naturally sensitive pathogens or by sensitizing intrinsic resistant strains [9]. Antibiotic adjuvants being in current research limelight, Chaudhuri M et al., have put an effort to combat resistance among Gram negative bacilli by evaluating novel adjuvant antimicrobial CSE 1034, a novel combination of Ceftriaxone+sulbactam+disodium edetate [10].
Keeping in view the above background, the present study was attempted to find the prevalence of MBL among various clinical isolates obtained from a multi specialty tertiary care hospital, Mumbai, India and to test in vitro susceptibility of the novel adjuvant antimicrobial CSE 1034 for these isolates and compare it with 3 other high end betalactam antibiotics.
Materials and Methods
Collection of clinical isolates
This prospective study was conducted in the Department of Microbiology of a multidisciplinary tertiary care centre in Mumbai. A total of 823 gram negative isolates were obtained out of 1120 isolates, sourced from blood, pus, respiratory secretions, tissue and urine which were collected from ICU and ward over the period of 8 months from March 2013 to October 2013. We first included the gram negative isolates which were resistant to carbapenems and phenotypically suggested of MBL production and phenotypically confirmed ESBL producers (141/823). ALL isolates that had mechanism of carbapenem resistant other than MBL production and those with more than one mechanism of resistance to betalactam antibiotics, for example ESBL+ AmpC, were excluded from the study. Repeat isolates were not included in the study. Samples were processed and identified as per standard methods [11] as well as Vitek-2 compact automated system.
Screening of isolates for ESBL and MBL production
Screening of isolates for ESBL production was performed as per CLSI guidelines [11]. Isolates exhibiting zone size ≤25 with ceftriaxone (30 μg), ≤22 for ceftazidime (30 μg) and ≤27 (30 μg) with cefotaxime were considered as possible ESBL producer. ESBL production was confirmed by disk potentiation test using ceftazidime (30 μg) and cefotaxime (30 μg) antibiotic disks with and without clavunalic acid (10 μg) and by double disc susceptibility test (DDST) [11]. Similarly, phenotypic detection of MBL among clinical isolates was carried out using imipenem (10 μg) and imipenem (10 μg) + EDTA (750 μg) discs as described by Yong et al., [12].
Antimicrobial susceptibility testing
The antimicrobial susceptibility testing was performed by the disc diffusion method as per CLSI guidelines in Vitek 2 compact automated system [11]. The disc of ceftriaxone+sulbactam+disodium edetate (55μg), cefoperazone + sulbactam (C+S) (45μg), piperacillin + tazobactam (pip-taz) (110μg) and meropenem (10 μg) were obtained from Hi-Media, Mumbai, India.
Results
Collection of clinical isolates and their identification
Among all the clinical isolates 73.48% (823/1120) were Gram negative isolates [Table/Fig-1]. However, among the 141 isolates included in the study, the predominant pathogen was K. pneumoniae (34.0%), followed by E. coli (33.3%).
Collection of clinical isolates from various clinical specimens
| S. No. | Samples | Clinical isolates |
|---|
| A. baumannii | E. coli | K. pneumoniae | P. aeruginosa | C. freundii | E. aerogenes | E. cloacae | P. vulgaris |
|---|
| 1 | Blood | 1 | 2 | 1 | 1 | - | - | - | - |
| 2 | Pus | 3 | 11 | 5 | 4 | - | - | - | - |
| 3 | Sputum | 3 | 2 | 9 | 1 | - | - | - | - |
| 4 | Tissue | 8 | 9 | 12 | 4 | - | - | 1 | 1 |
| 5 | Urine | 3 | 23 | 18 | 11 | 1 | 2 | - | - |
| 6 | Tracheal secretion | - | - | 1 | - | - | - | - | - |
| 7 | ET | 2 | - | 2 | - | - | - | - | - |
| Total (141) | | 20 | 47 | 48 | 21 | 1 | 2 | 1 | 1 |
Diversity of ESBL and MBL
All these isolates were subjected to screening for ESBL and MBL of which 35.46 % (50/141) were ESBL producers and 64.53 % (91/141) were confirmed to be MBL producers. The maximum number of ESBL producers belonged to E. coli followed by K. pneumoniae. The overall prevalence of MBL production was 11 percent (n=91) and maximum number of MBL producers belonged to K. pneumoniae followed by Pseudomonas aeruginosa. The detail distribution of ESBL and MBL in various clinical samples is shown in [Table/Fig-2].
Sample wise distribution of ESBL and MBL in different clinical isolates
| Source of Sample | A. baumanii | E. coli | K. pnemoniae | P. aeroginosa | C. freundii | E. aerogenes | E. cloacae | P. vulgaris |
|---|
| ESBL | MBL | ESBL | MBL | ESBL | MBL | ESBL | MBL | MBL | MBL | MBL | ESBL |
|---|
| Blood | 01 | | 02 | | | 01 | | 01 | | | | |
| Pus | | 03 | 09 | 02 | | 05 | | 04 | | | | |
| Sputum | | 03 | 01 | 01 | 03 | 06 | | 01 | | | | |
| Tissue | | 08 | 08 | 01 | 06 | 06 | | 04 | | | 01 | 01 |
| Urine | | 03 | 17 | 06 | 02 | 16 | | 11 | 01 | 02 | | |
| Tracheal scretion | | | | | | 01 | | | | | | |
| ET | | 02 | | | | 02 | | | | | | |
| Total (141) | 01 | 19 | 37 | 10 | 11 | 37 | | 21 | 01 | 02 | 01 | 01 |
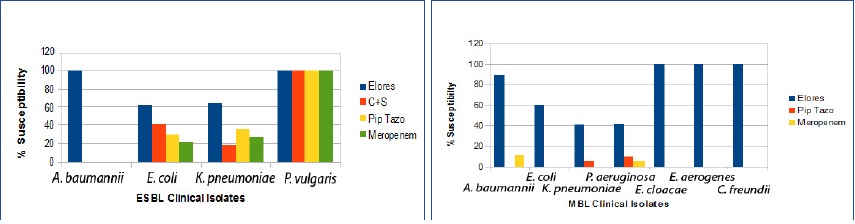
According to antibiogram results presented in [Table/Fig-3], CSE-1034 was found to be most sensitive antibacterial agent against ESBL producing organisms. For, all ESBL producers, A. baumannii isolates were most susceptible to CSE-1034 whereas 64% and 62% isolates of K. pneumoniae and E. coli respectively were susceptible to CSE-1034 and 18% and 16% were resistant to CSE-1034.
Antimicrobial susceptibilities of clinical isolates as per table
| Clinical isolates | Charact-erization | CSE-1034 | C+S | Pip+Taz | Meropenem |
|---|
| S | I | R | S | I | R | S | I | R | S | I | R |
|---|
| A. baumannii | ESBL | 100 | - | - | - | - | 100 | - | - | 100 | - | 100 | - |
| MBL | 89 | 11 | - | - | 47 | 53 | - | 5 | 95 | 11 | 26 | 63 |
| E. coli | ESBL | 62 | 22 | 16 | 41 | 32 | 27 | 30 | 22 | 49 | 22 | 19 | 59 |
| MBL | 60 | 10 | 30 | - | - | 100 | - | - | 100 | - | - | 100 |
| K. pneumoniae | ESBL | 64 | 18 | 18 | 18 | 36 | 45 | 36 | - | 64 | 27 | - | 73 |
| MBL | 41 | 32 | 27 | - | 16 | 84 | 5 | - | 95 | - | - | 100 |
| P. aeruginosa | ESBL | - | - | - | - | - | - | - | - | - | - | - | - |
| MBL | 42 | 51 | 7 | - | 19 | 81 | 10 | - | 90 | 5 | 10 | 86 |
| E. cloacae | ESBL | - | - | - | - | - | - | - | - | - | - | - | - |
| MBL | 100 | - | - | - | - | 100 | - | - | 100 | - | - | 100 |
| E. aerogenes | ESBL | - | - | - | - | - | - | - | - | - | - | - | - |
| MBL | 100 | - | - | - | - | 100 | - | - | 100 | - | - | 100 |
| C. freundii | ESBL | - | - | - | - | - | - | - | - | - | - | - | - |
| MBL | 100 | - | - | - | - | 100 | - | - | 100 | - | - | 100 |
| P. vulgaris | ESBL | 100 | - | - | 100 | - | - | 100 | - | - | 100 | - | - |
| - | - | - | - | - | - | - | - | - | - | - | - |
Interestingly among the tested drugs, CSE-1034 was found to be most susceptible against MBL producing isolates. Approximately, 89%, 60%, 42% and 41% isolates of A. baumannii, E. coli, P. aeruginosa and K. pneumoniae, respectively were susceptible to CSE-1034 [Table/Fig-4]. None of the isolates of A. baumannii were resistant to CSE-1034 whereas 30 %, 27% and 7 % isolates of E. coli, K. pneumoniae, and P. aeruginosa, respectively were resistant to CSE-1034 and 11%, 10%, 32% and 51 % isolates of A. baumannii, E. coli, K. pneumoniae and P. aeruginosa, respectively showed intermediate response to CSE-1034 [Table/Fig-5].
Comparative Susceptibility Percentage of different drugs on clinical isolates
Comparative resistance of different drugs in ESBL and MBL producing clinical isolates
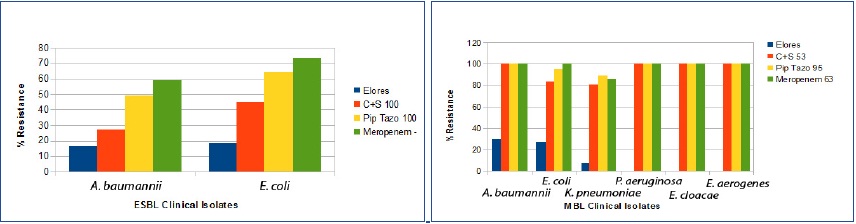
A particular important feature was that isolates of MBL producing Enterobacter spp, C. freundii were 100 % susceptible to CSE-1034 but were resistant to all other comparator drugs.
Discussion
β-lactam antibiotics are largely used antibiotics to treat infections. The incidence of ESBL producing E.coli and K. pneumonia has increased dramatically all over the country. As per SMART program 2007 ESBL rates in India for E.coli was 79%, and that for K pneumonia was 69.4% and for K oxytoca 100% [13]. When precious lives are at stake, carbapenems are the last resort. However resistance to carbapenems are up-surging, especially among Enterobacteriaceae members, In one study, carbapenem resistance has increased from 0% in 2006 to 8% in 2009 in ICU blood cultures of a tertiary care hospital in Mumbai [3]. In a study from New Delhi, India 79% of the Klebseilla pneumonia isolates were MBL producers [4]. The prevalence of MBL producers in our study was 11% which is low as compared to the 42.1 % in tertiary care hospital, Kolkata 56 % in MGM Medical College and Hospital, Kamothe, Navi Mumbai, India [5,6].
Now we ponder on options for such infections, with only colistin and tigecycline in hand, probably a re-shuffle of the existing ones would checkmate the MDR pathogens. As per a systemic evaluation by Falagas et al., a combination antibiotic treatment may be considered the optimal option for severely ill patients with severe infection [14]. There are a number of newer molecules in pipe line awaiting proof of safety and efficacy and are in phase 2 or 3 studies. Ceftolozane/tazobactam, Ceftazidime - avibactam, Ceftaroline - avibactam, Imipenem/MK-7655, Plazomicin (ACHN-490), Eravacycline (TP-434), Brilacidin (PMX-30063) are in queue [15]. Another strategic initiative is development of adjunctive antibiotic therapies i.e. novel combinations of antimicrobial drugs with adjuvant molecules lacking intrinsic antibiotic activity, which overcome microbial resistance [16].Antibiotic adjuvants can function by (i) affecting a vital physiological bacterial function; (ii)bacterial inhibition of antibiotic resistance elements; (iii) enhancement of the uptake of the antibiotic through the bacterial membrane; (iv) direct blocking of efflux pumps;and (iv) changing the physiology of resistant cells function i.e. alteration in biofilm formation [9].
Chaudhuri M et al., compared and evaluated the efficacy and synergistic effect of a third generation cephalosporin - ceftriaxone and a potent beta lactamase inhibitor, sulbactam in different combination ratios and also evaluated the effect of addition of EDTA, a potent class B metallo betalactamse inhibitor and its salts in this potential combination and concluded that ceftriaxone +sulbactam in the ratio of 2:1 along with EDTA disodium (3 mg/ml) [CSE1034] lowers MIC to >8 fold and possess synergy against the most ESBL producing micro organisms [10]. CSE1034 is effective against MDR pathogen producing ESBLs, MBLs like NDM-01, increases cell permeability while working on cell impermeability mechanism of MDRs, regulate ‘Efflux pump over expression’, breaks bacterial ‘Biofilms’, prevents ‘Transfer of resistant plasmid’ and hence the spread of resistance is controlled [10,17–22].
Our study showed 64 to 100% susceptibility to CSE-1034 towards ESBL producing isolates. Our data showed that all ESBL positive isolates of P. vulgaris were found to be susceptible to CSE-1034, C+S, Pip Tazo and Meropenem. Earlier studies from India have demonstrated the reduced susceptibility of C+S, Pip Tazo and meropenem to ESBL producing organisms and higher susceptibility to CSE-1034 [23–27]. Susceptibility results revealed that all the MBL producing isolates of A. baumannii were found to be resistant to almost all antibiotics except CSE-1034 (89% susceptible). Earlier study from India demonstrated 89% resistance to meropenem in MBL producing A. baumannii [8]. A similar trend was observed in other strains but with lower susceptibility rates. All the MBL positive isolates of E. cloacae, E. aerogenes and C. freundii were observed to be resistant to C+S, Pip Tazo and meropenem but sensitive to CSE-1034, indicating again the usage of CSE-1034 in MBL positive isolates. These results corroborates with other Indian reports where MBL producing K. pneumoniae, E. coli, A. baumannii and P. aeruginosa were found to have lesser susceptibility of C+S, Pip Tazo and meropenem and greater susceptibility to CSE-1034 [23–27].
In a randomized, open-label, multicenter study of CSE1034 (Elores) versus ceftriaxone in the treatment of LRTIs and UTIs conducted on 297 patients, the overall, clinical cure rate was high in the group of patients treated with Elores in comparison to ceftriaxone [27]. Elores was resistant only to those strains which were positive with TEM-50, OXA-11 and CTXM-9. Chaudhary M and Payasi A et al., compared clinical and bacteriological efficacy as well as tolerability of ceftriaxone-sulbactam with adjuvant disodium edentate (Elores) in the treatment of skin and skin structure infections (SSSIs) and bone and joint infections (BJIs) and found that 80.33% of the total patient population was clinically cured with CSE1034 [28].
Conclusion
With increasing resistance to the BL-BLI combinations and carbapenems used to treat infections caused by variety of resistant gram negative organisms, we would recommend inclusion of Ceftriaxone+sulbactam+disodium edetate (CSE1034), a novel Antibiotic Adjuvant Entity (AAE) in routine antibiotic sensitivity panel for gram negative isolates to generate the local data and this may be considered as an alternate, carbapenem sparer in ESBL producing pathogens and a promising option for MBL producing pathogens.