Seroprevalence of Hepatitis B, Hepatitis C and Human Immunodeficiency viruses amongst Injecting Drug Users in Mumbai,India
Avantika Shukla1, Anuradha Sharma2
1Consultant Microbiologist, Department of Microbiology, Karuna Hospital, Borivali (East), Mumbai, India.
2Associate Professor, Department of Microbiology, Faculty of Dentistry, Jamia Millia Islamia, New Delhi, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Anuradha Sharma, Associate Professor, Department of Microbiology, Faculty of Dentistry, Jamia Millia Islamia, Jamia Nagar, New Delhi-110025, India.
Phone: 9968050032 /7838230809,
E-mail: d_anuradha@yahoo.com,asharma2@jmi.ac.in
Dear Sir,
Injecting drug users (IDUs) are prone to infections caused by pathogens like hepatitis B virus, hepatitis C virus and human immunodeficiency virus. Their transmissions are primarily parenteral, which occur through the sharing of contaminated injection equipments.
This study was carried out from August 2007 to October 2007 at the Department of Microbiology, T. N. Medical College and BYL Nair Charitable Hospital, Mumbai, India, after obtaining the approval of the institutional ethics committee.
The sample size and the study design were the same as were followed at NACO identified sentinel sites at Mumbai, for the annual HIV sentinel surveillance round. Two hundred and fifty blood samples were collected by using an outreach strategy. Sample size was determined by the formula, n=Z2 p (1-p)/d2, where n= sample size, z= 1.96 for a 95% confidence limit, p= prevalence rate and d=degree of precision [1]. The target group comprised of first time visitors at the surveillance round, who had injected at least once in the last six months and were identified by a drug de-addiction centre. Testing for anti-HIV antibodies was done by using Microlisa (J.Mitra and Co., New Delhi) and Pareekshak ½ Triline (Bhat Biotec India (P) Ltd. Bangalore, Karnataka), as per strategy II of NACO guidelines. For anti-HCV antibodies, INNOVA HCV ELISA Kit test ( Span Diagnostics, Surat, India ) was used and for HBsAg, ERBA LISA hepatitis B kit (Transasia Biomedicals Ltd. Mumbai, India) was used. The positive samples were confirmed by retesting the respective ELISA tests.
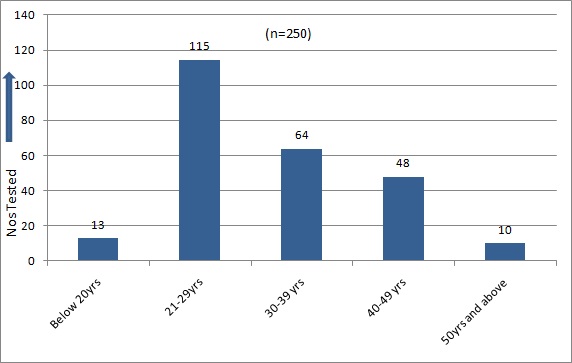
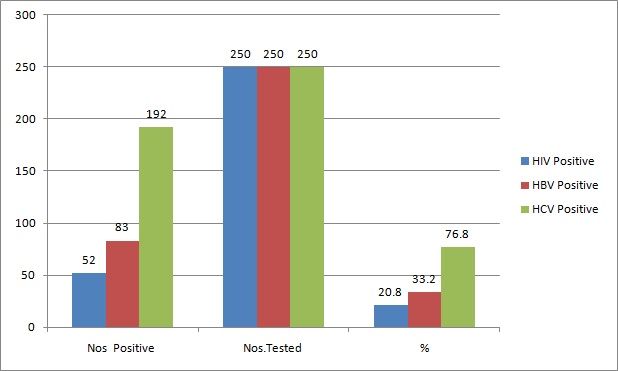
The target population was urban in locale. 76% were non-migrants and 54.4% were illiterate, and only 2.4% were graduates. Most of them were from agricultural background. One hundred and fifteen (46%) of the IDUs were of the age group of 21-29 years [Table/Fig-1]. The male to female ratio was 100: 6.8. The first injecting drug abuse had occurred at the age of 18 years, and the ages of IDUs ranged from 18-67 years [Table/Fig-1]. Fifty Two (20.8%) tested positive for antibodies to HIV 1, 192 (76.8%) were positive for antibodies to HCV and 83(33.2%) were HBsAg positive [Table/Fig-2]. HIV-HCV co-infection was detected in 16% cases (p-value: 0.8536 ), HIV-HBV co-infection was detected in 7.8% cases (p-value : 0.6149) and HIV and HBV co-infection was seen in 7.2% cases (p-value: 0.6149 ). HIV-HBV-HCV co-infection was seen in 6% of the IDUs. Co-infections were not statistically significant [Table/Fig-2,3].
20.8% HIV infection seen amongst IDUs was similar to that seen in a previous study [2], though 30% and 59.6% infection had also been reported previously [3-5].Only HIV 1 infection was detected, although there had been reports on HIV 2 infection seen among IDUs in Manipur, India, being a pattern II country for the spread of HIV, the major mode of HIV transmission is heterosexual (70-75%) and therefore, HIV in IDUs quickly spreads to the general population.
33.2% HbsAg reactivity seen in the present study was similar to those seen in studies done in 2000 and 2001, in which the HBsAg positivity in IDUs was found to be 35.3% [6]. Most of the HBV infections occur as a result of sexual activity, intravenous drug use, or occupational exposure. Less common causes include household contact, haemodialysis, and receipt of solid organs or blood products. No clear risk factors were identified in 20% to 30% of infected individuals.
A high HCV reactivity of 76.8% seen in the present study, was similar to 76.9% in Garfein’s study [2]. In a previous study in 2004, 61.2% HCV reactivity was reported from Mumbai [5]. Factors which are associated with HCV mono-infection and HIV-HCV co-infection are: longer durations of injecting drug use, use of multiple injection drugs and sharing injection equipments and containers.
Age distribution of IDU’s
HIV, HBV and HCV Co-infections in IDU’s (n=250)
| anti-HIV , HbsAg , anti- HCV Reactivity (n=250) |
| | HIV +ve | HIV -ve | Total |
| HBsAg +ve | 18 | 65 | 83 |
| HBsAg -ve | 34 | 133 | 167 |
| anti-HCV +ve | 40 | 152 | 192 |
| anti-HCV --ve | 12 | 46 | 58 |
| Total | 52 | 198 | 250 |
HIV, HBV, HCV Reactivity in IDU’s (n=250)
Conclusion
Lower rates of HIV and higher rates of HBV and HCV infection seen among IDUs indicated that HBV and HCV were more transmissible than HIV among IDUs, due to their unsafe injection practices. HIV positive IDUs who were co-infected with HCV /HBV were more likely to face disease progression to chronic liver disease and cirrhosis. In such cases, ART becomes problematic, due to hepatotoxixity. Hence, counselling should be done, to reduce the risk of household, sexual and needle-sharing transmissions and for promoting the need of hepatitis B vaccinations.
[1]. Guidelines for measuring National HIV prevalence in population based surveys: UNAIDS/WHO working group on Global HIV/AIDS and STI surveillanceSwitzerlandGeneva,:15-16. [Google Scholar]
[2]. Garfein Richard S, Vlahov David, Galai Noya, Doherty Meg C, Nelson Kenrad E, Viral Infections in Short-Term Injection Drug Users : The Prevalence of the Hepatitis C,Hepatitis B, Human Immunodeficiency and Human T-lymphotropic viruses.American Journal of Public Health 1996 86(5):655-61. [Google Scholar]
[3]. KhS Devi, N Brajachand, HL Singh, YM Singh, Co-infection by human immuno deficiency virus, hepatitis B and hepatitis C virus in injecting drug usersJ Commun Dis 2005 37(1):73-7. [Google Scholar]
[4]. B Borkakoty, J Mahanta, HK Das, Co-infection of HIV, HCV, HBV and the associated risk behaviors among injection drug users in two north-eastern states of India. 4th International AIDS Society Conference on HIV Pathogenesis, Treatment and Preventio (IAS 2007). 2007 Sydney, Australia [Google Scholar]
[5]. K Saraswati, A Dutta, A Study of Human Immunodeficiency Virus And HCV infections in Intravenous Drug Users in MumbaiIJMM. 2007 25:174--5. [Google Scholar]
[6]. MK Saha, S Chakrabarti, S Panda, TN Naik, B Manna, A Chatterjee, Prevalence of HCV & HBV infection amongst HIV seropositive intravenous drug users and their non-injecting wives in Manipur, India.Indian J Med Res 2000 111:37-9. [Google Scholar]