Modern dentistry aims to restore the comfort and health of the stomatognathic system. Dental implants have emerged as a promising option for this purpose. Osseointegration forms the basis of implant success. Studies have been conducted to establish the criteria for success and failure of osseointegration and factors affecting osseointegration [1,2]. Traditionally, an unloaded healing period was considered essential for the achievement of osseointegration of dental implants [3]. Now in implant dentistry, advanced treatment protocols such as early or immediate loading are frequently used to reduce treatment time but this poses new demands on both the primary and secondary implant stability. Implant stability is defined as the capacity of the implant to withstand loading in axial, lateral, and rotational directions [4]. Primary stability is mainly dependent on the mechanical characteristics of the original bone like its local quality and quantity, the type of implant used including its geometry, diameter, length & surface characteristics, and the surgical techniques employed [5,6].Secondary implant stability represents enhancement of the stability as a result of peri-implant bone formation through gradual bone remodeling and osteoconduction, with the possibility of new bone formation at the implant-bone interface and influenced by the implant surface characteristics [7,8]. Contemporary knowledge indicates that the degree of micromotion at the bone-implant interface during initial healing is of utmost importance in achieving good secondary stability [9–11].
Several measures have been proposed to improve and accelerate osseous healing of endosseous implants. Platelet-Rich-Plasma (PRP) has been suggested to enhance the healing of bone grafts and to enhance the integration of implants into bone, as activated platelets release autogenous growth factors (GFs) into the wound healing site [12–15]. Platelet-Rich Plasma (PRP) is defined as a portion of the plasma fraction of autologous blood having a platelet concentration above baseline. PRP also has been referred to as platelet-enriched plasma, platelet rich concentrate, platelet releasate and autologous platelet gel. Platelet releasates have been used to treat wound since 1985. It serves as a growth factor agonist and has both mitogenic and chemotactic properties. It contains a high level of platelets and a full complement of clotting and growth factors [15]. Radiographic examination of marginal bone around the implant and evaluation of the mobility of the implant are among the most reliable methods to evaluate osseointegration clinically [16].
Therefore, the main objective of this RCT was to assess the effects of PRP and different implant surface topography on implant stability and bone around immediately loaded dental implants. Hypothesis was postulated that patients who received implants treated with PRP and square thread form implants would have significantly higher implant stability and bone levels than those who received implants without PRP treatment at baseline, one and three months post implant placement.
Materials and Methods
A double blinded, randomized controlled trial (RCT) was done including the subjects selected from the Out Patient Department of Prosthodontics and Crown and Bridge from February 2012 to November 2013. The inclusion criteria were: (a) Subjects with missing mandibular posterior teeth, (b) Subjects with adequate interocclusal clearance & adequate mesio-distal (M-D) space in edentulous area, (c) Subjects who had given signed informed consent. Exclusion criteria were: (a) Subjects with smoking habits, (b) Subjects with immunocompromised state and debilitating diseases, (c) Subjects on medication known to interfere with wound and bone healing and (d) Subjects with parafunctional habits were also excluded from the study. Ethical approval was obtained from the Institutional Ethics Committee with approval no.PGIDS/2012/IEC/22 and written informed consent was obtained from all selected subjects. The subjects were divided into two groups: Group I- without PRP i.e. implants were placed by following conventional single stage surgical protocol and immediately loaded within two weeks, Group II- with PRP i.e. implants were placed after dipping in activated PRP and randomly assigned them to receive implant supported prosthesis with PRP or without PRP surface treatment. Within these two groups, all subjects were randomly divided into following subgroups based on thread form of implant used:
Subgroup IA- Non-PRP Square thread form.
Subgroup IB- Non-PRP Reverse buttress thread form.
Subgroup IIA- PRP Square thread form.
Subgroup IIB- PRP Reverse buttress thread form.
Initially, 42 sites were selected for the study, but during treatment planning procedure, five subjects were excluded after diagnostic impressions and radiographic investigations as bone was deficient in edentulous area for implant placement, three subjects dropped out after CT scan procedure and four subjects were excluded from the study after implant placement & temporization due to implant failure. So, a total of 30 implant sites were included in the study. 15 implants were placed in each group and eight implants in each square thread form subgroup and seven implants in each reverse buttress thread form subgroup. The study population selected was North Indian population with 43.3% males and 56.7% females. The mean age was 33.93 ± 11.25, with a range of 18 to 56 years. After an explanation of the proposed study criteria, including alternate treatment options, potential risks and benefits, a signed informed consent was obtained from all the subjects prior to the implant placement.
Control Group
Subjects randomized to the control group received implants in mandibular posterior region with square and reverse buttress thread form Titanium implants with RBT coating (BIOHORIZONS, USA) following conventional single stage surgical protocol.
Experimental Group
Subjects randomized to the experimental group received implants in mandibular posterior region with square and reverse buttress thread form Titanium implants with RBT coating (BIOHORIZONS, USA) which were treated with PRP; following single stage surgical protocol.
Preparation of PRP
The patients were subjected to complete haemogram analysis. Before starting the surgical procedure, for experimental group, patient’s 5 ml venous blood was drawn from the antecubital vein in sterile Vacutainer containing 1 ml Citrate Phosphate Dextrose- Adenine (CPDA) as anticoagulant and centrifuged at 2400 rpm for 10 minutes. After the first centrifugation, two layers were seen clearly in the Vacutainer. The upper yellow layer was consisting of platelet rich and poor plasma and lower red layer was consisting of erythrocytes and leukocytes. Then the complete upper yellow layer and lower red layer’s top 1-2 mm part was transferred into plain Vacutainer. After the second centrifugation at 3600 rpm for 15 minutes, approximately 1ml was plasma rich from platelets at bottom of Vacutainer and the upper rest was plasma poor from platelets. The part of platelet poor plasma was discarded and remaining plasma at bottom was stored in platelet agitator till its use.
Surgical procedure
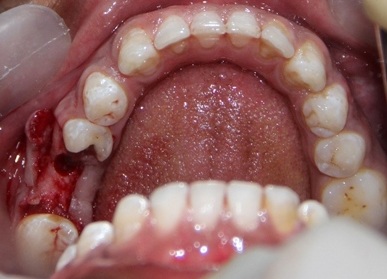
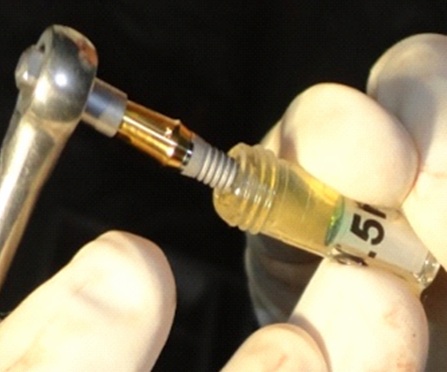
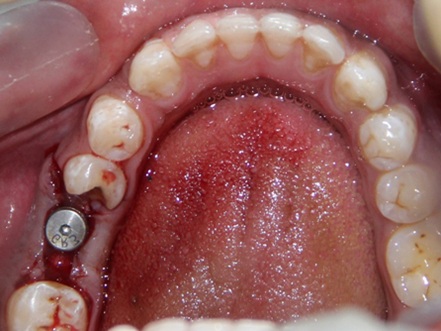
Osteotomy preparation was done following standard surgical protocols with adequate irrigation [Table/Fig-1]. For experimental group, the PRP was activated with 10% CaCl2 solution and implant surface was treated with activated PRP by dipping in it and by avoiding any contact with the walls of the container [Table/Fig-2]. Then implant was placed into the prepared osteotomy and healing abutment was placed [Table/Fig-3] and flap closure done. For control group, implants were placed without PRP treatment.
Osteotomy site preparation done
Implant surface treated with activated PRP
Implant placement done with healing abutment in the prepared osteotomy site
Temporization and final prosthesis
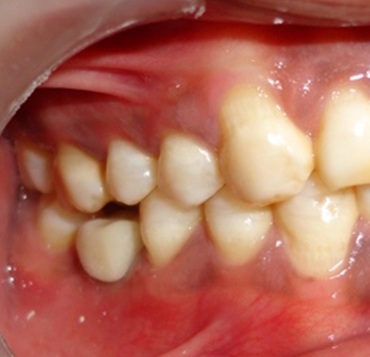
The temporary crown was fabricated with tooth moulding auto polymerizing acrylic resin (DPI) by indirect technique and luted with temporary cement zinc oxide non eugenol (Relyx Temp NE, 3M ESPE) within two weeks of implant placement. The temporary prosthesis was kept out of occlusion with opposing tooth [Table/Fig-4]; immediate non-functional occlusal loading was done. Final prosthesis was given after three months of implant placement. The healing abutment was replaced with implant abutment and final impression was made with Vinyl Polysiloxane putty and light body impression material (Express STD; 3M ESPE, USA). Final cast was prepared with implant analog. Porcelain (DENTSPLY, USA) fused to metal (Wirocar plus; BEGO, USA) prosthesis was fabricated and cemented [Table/Fig-5] with zinc phosphate cement (SUPER CEMENT SHOFU).
Temporization done and prosthesis kept out of occlusion
Final prosthesis cementation done
Outcome measures
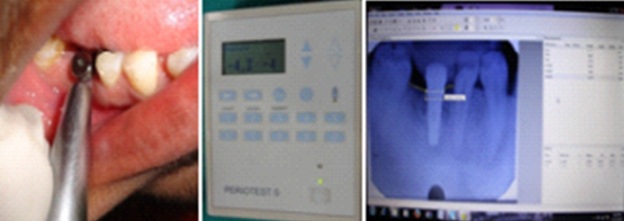
The implant stability and bone levels were measured at baseline (two weeks of implant placement), one month and three months. The implant stability was measured with Periotest by placing handpiece perpendicular to the longitudinal axis of the implant and orthoradially to the arch, holding the handpiece parallel to the floor at about 2mm distance [Table/Fig-6]. Readings were recorded until the device registered the same value three consecutive times. Then the PTVs (range -8 to + 50) were analysed between all the groups. The bone levels were assessed with Intra Oral Periapical radiographs (IOPA x –rays) taken with paralleling technique using XCP-Rinn apparatus and angulation was standardized by making putty occlusal jig. The x-rays were digitalized. Using the apical corner of the implant collar as the reference line and the lowest point of marginal bone around the implant as the bone level, the distance was measured to the nearest 0.01 mm with the Digimizer Image Analysis Tool (MedCalc Software, version 4.2.5.0, [Table/Fig-2]). Bone levels were measured on the mesial and distal aspects of the implants. A positive value indicated a level coronal to the reference line and a negative value indicated a level apical to the reference line. The readings were recorded at baseline, one month and three months for all groups; these readings were then analysed statistically.
Implant stability measured with Periotest and Bone level measurements done using Digimizer Image Analysis software
Statistical Analysis
The readings obtained were analysed using IBM SPSS Statistics (Statistical Presentation System Software) for Windows, version 19.0 (SPSS Inc: New York) and Kruskal-Wallis, Mann-Whitney and Wilcoxon tests were used to evaluate the inter-group and intra-group differences respectively. The values of implant stability and bone levels were compared with baseline at one month and at three months within same group; and between groups, values were compared at baseline, one month and three months. The p-value two tailed was taken significant at p< 0.05 and highly significant at p< 0.01.
Results
Out of the 30 implants which were placed for the study, 13 implants (43.3%) were placed in males and 17 implants (56.7%) were placed in females. At baseline, a statistically highly significant difference (p=0.003) was noticed in implant stability based on Periotest values between PRP treated and non treated groups but statistically non-significant difference was found at one month (p=0.107) and three months (p=0.153, [Table/Fig-7]). The effect of PRP on bone height changes were not statistically significant at all time intervals i.e. baseline, one month and three months (p>0.05, [Table/Fig-8,9]). The effect of thread form (square and reverse buttress) on implant stability in implants with and without PRP treatment was not statistically significant (p>0.05, [Table/Fig-7]), however, a square thread form surface topography of implant was associated with less bone resorption clinically as compared to reverse buttress thread form from baseline to three months though statistically insignificant [Table/Fig-8,9]. A synergistic effect of PRP and square thread form was observed on improved implant stability (p>0.05) and bone levels (p>0.05, [Table/Fig-10]); however, no such effect is seen with PRP and reverse buttress thread form (p<0.05, [Table/Fig-10]). No variation was observed in implant stability and bone levels with gender and site of implant placement.
Comparison of Periotest values (PTV) at Baseline, One month & Three months of implant placement between Group I& II and Subgroups IA, IB, IIA & IIB
| Groups & Subgroups | Baseline (PTV0) | p-value† | One Month (PTV1) | p-value | Three Month (PTV3) | p-value |
|---|
| Mean ± SD | Mean ± SD | Mean ± SD |
|---|
| Group I* | 1.00 ± 6.64 | 0.003‡ | (-) 0.07 ± 4.7 | 0.107 | (-) 1.67 ± 4.05 | 0.153 |
| Group II | (-) 4.47 ± 2.23 | (-) 2.13 ± 5.14 | (-) 2.33 ± 6.15 |
| Subgroup IA | 0.00 ± 5.78 | 0.225 | (-)1.12 ± 5.03 | 0.31 | (-) 2.00 ± 4.41 | 0.75 |
| Subgroup IB | 2.14 ± 7.82 | 1.14 ± 4.34 | (-) 1.28 ± 3.90 |
| Subgroup IIA | (-) 4.75 ± 0.88 | 0.75 | (-)1.00 ± 6.69 | 0.167 | (-) 1.00 ± 8.05 | 0.197 |
| Subgroup IIB | (-) 4.14 ± 3.23 | (-) 3.43 ± 2.37 | (-) 3-85 ± 2.73 |
| Subgroup IA | 0.00 ± 5.78 | 0.05 | (-)1.12 ± 5.03 | 0.671 | (-) 2.00 ± 4.41 | 0.63 |
| Subgroup IIA | (-) 4.75 ± 0.88 | (-)1.00 ± 6.69 | (-) 1.00 ± 8.05 |
| Subgroup IB | 2.14 ± 7.82 | 0.023 | 1.14 ± 4.34 | 0.046 | (-) 1.28 ± 3.90 | 0.136 |
| Subgroup IIB | (-) 4.14 ± 3.23 | (-) 3.43 ± 2.37 | (-) 3-85 ± 2.73 |
* Mean, Standard Deviation and p-values- ‘two tailed’ for Periotest values (PTV) taken at baseline (Two weeks of implant placement), one month and three months of implant placement. †First versus second measurement. ‡Statistically significant (p-value <0.05)
Comparison of Bone levels on Mesial side (BLM) at Baseline, one month & three months of implant placement between Group I & II and Subgroups IA, IB, IIA & IIB
| Groups & Subgroups | Baseline (BLM0) | p-value† | One Month (BLM1) | p-value | Three Months (BLM3) | p-value |
|---|
| Mean ± SD (mm) | Mean ± SD (mm) | Mean ± SD (mm) |
|---|
| Group I* | 1.79 ± 0.37 | 0.398‡ | 1.05± 0.30 | 0.59 | 0.76± 1.07 | 0.443 |
| Group II | 1.74± 0.76 | 1.24± 0.93 | 1.15± 0.88 |
| Subgroup IA | 1.69± 0.33 | 0.398 | 1.09± 0.38 | 0.499 | 0.85± 1.42 | 0.866 |
| Subgroup IB | 1.89± 0.41 | 0.99± 0.19 | 0.67± 0.56 |
| Subgroup IIA | 1.61± 0.71 | 0.463 | 1.07± 1.05 | 0.866 | 0.85± 1.41 | 0.398 |
| Subgroup IIB | 1.90± 0.84 | 1.43± 0.82 | 1.36± 0.84 |
| Subgroup IA | 1.69± 0.33 | 0.674 | 1.09± 0.38 | 0.958 | 0.85± 1.42 | 0.834 |
| Subgroup IIA | 1.61± 0.71 | 1.07± 1.05 | 0.85± 1.41 |
| Subgroup IB | 1.89± 0.41 | 0.482 | 0.99± 0.19 | 0.482 | 0.67± 0.56 | 0.179 |
| Subgroup IIB | 1.90± 0.84 | 1.43± 0.82 | 1.36± 0.84 |
*Mean, Standard Deviation and p-values- ‘two tailed’ for Bone levels on mesial side measured in mm taken at baseline (at time of implant placement), one month and three months of implant placement. †First versus second measurement. ‡Statistically non-significant (p-value >0.05)
Comparison of Bone levels on Distal side (BLD) at Baseline, one month & three months of implant placement between Group I&II and Subgroups IA, IB, IIA & IIB
| Groups & Subgroups | Baseline (BLD0) | p-value† | One Month (BLD1) | p-value | Three Months (BLD3) | p-value |
|---|
| Mean ± SD (mm) | Mean ± SD (mm) | Mean ± SD (mm) |
|---|
| Group I* | 1.76 ± 0.52 | 0.983‡ | 1.21± 0.57 | 0.82 | 0.91± 1.06 | 0.694 |
| Group II | 1.67± 0.84 | 1.11± 0.85 | 1.10± 0.93 |
| Subgroup IA | 1.62± 0.63 | 0.237 | 1.03± 0.58 | 0.176 | 0.91± 1.28 | 0.735 |
| Subgroup IB | 1.93± 0.33 | 1.41± 0.53 | 0.91± 0.83 |
| Subgroup IIA | 1.38± 0.79 | 0.063 | 0.69± 0.77 | 0.058 | 0.67± 0.94 | 0.063 |
| Subgroup IIB | 2.01± 0.82 | 1.58± 0.71 | 1.56± 0.69 |
| Subgroup IA | 1.62± 0.63 | 0.753 | 1.03± 0.58 | 0.462 | 0.91± 1.28 | 0.345 |
| Subgroup IIA | 1.38± 0.79 | 0.69± 0.77 | 0.67± 0.94 |
| Subgroup IB | 1.93± 0.33 | 0.749 | 1.41± 0.53 | 0.306 | 0.91± 0.83 | 0.142 |
| Subgroup IIB | 2.01± 0.82 | 1.58± 0.71 | 1.56± 0.69 |
*Mean, Standard Deviation and p-values- ‘two tailed’ for Bone levels on mesial side measured in mm taken at baseline (at time of implant placement), one month and three months of implant placement. †First versus second measurement. ‡Statistically non-significant (p-value >0.05)
Intra-group comparison of Periotest values and Bone levels on Mesial & Distal side at Baseline versus one month & Baseline versus three months of implant placement between Group I&II and Subgroups IA, IB, IIA & IIB
| Groups & Sub groups | Baseline versus One Month (p-values)* | Baseline versus Three Months (p-values)† |
|---|
| Periotest values | Bone levels on Mesial side | Bone levels on Distal side | Periotest values | Bone levels on Mesial side | Bone levels on Distal side |
|---|
| Group I | 0.476 | 0.001‡ | 0.001 | 1.32 | 0.005 | 0.003 |
| Group II | 0.049 | 0.005 | 0.009 | 0.156 | 0.003 | 0.008 |
| Subgroup IA | 0.481 | 0.012 | 0.012 | 0.207 | 0.208 | 0.123 |
| Subgroup IB | 0.735 | 0.018 | 0.018 | 0.351 | 0.018 | 0.018 |
| Subgroup IIA | 0.04 | 0.161 | 0.069 | 0.131 | 0.036 | 0.093 |
| Subgroup IIB | 0.461 | 0.018 | 0.063 | 0.589 | 0.028 | 0.043 |
*p-values- ‘two tailed’ for Periotest values and Bone levels on mesial & distal side measured in mm taken at baseline and one month of implant placement within same group. †p-values- ‘two tailed’ for Periotest values and Bone levels on mesial & distal side measured in mm taken at baseline and three months of implant placement within same group. ‡Statistically significant (p-value <0.05)
Discussion
To best of our knowledge, this is the first RCT specifically conducted to evaluate the effects of Platelet-Rich-Plasma (PRP) and implant surface topography together in humans. Hypothesis was postulated that PRP and square thread form implants improve implant stability and bone formation. In this study, providing treatment with implants treated with PRP and square thread form corroborated this hypothesis.
The stability of implants is high on the day of placement; marked decrease in implant stability was noticed at one month based on Periotest values compared to baseline. However, at three months there was an increase in implant stability at statistically significant level [17,18]. Comparison within PRP and Non-PRP groups, showed a statistically significant difference in implant stability at baseline whereas, at one month and three months, no significant difference was noticed [Table/Fig-7]. These findings are in accordance with the results of the study by the authors, Peev S et al., [19] in which improved stability of immediate loaded implants in the period between second and sixth week of their loading were observed with application of PRP. A significant change in bone levels was observed from baseline to one month and three months, so, effect of PRP on bone formation was found to be non-significant, these results contradict the results of the study by authors Anitua EA, [12] Anand U, [20] & Manimaran et al., [21] and are in accordance with the results of the study by Froum SJ et al., [22] Garcia RV et al., [23] and El –marssafy et al., [24] who suggested that PRP does not enhance bone formation around dental implants though they have used different types of implants in maxillary posterior areas. Similar implant stability was achieved with square and reverse buttress thread form implants. These findings are in accordance with Vidyasagar L et al., [25] who also achieved similar primary stability in different implant designs in pig ribs. Also, no significant interaction was seen between two thread forms and bone levels in both PRP treated and non treated cases.
In PRP square thread form subgroup, implant stability was found to be decreased at one month and again increased at three months. However, no significant bone loss was observed at baseline, one month and three months except at three months on mesial side in which bone loss was observed which may be due to direction of occlusal forces. Therefore, a synergistic effect of PRP and square thread form was observed on both implant stability and bone levels. Within PRP reverse buttress thread form subgroup, no such effect was seen. Also, in this study, no statistically significant interaction was noticed between Gender and site of implant placement to PRP and implant surface topography.
Molecular and cellular contributions to endosseous implant osseointegration include factors that affect bone formation and bone adaptation [26]. The bone formation at implant bone interface can be attributed to three processes: osteoconduction, osteogenesis and osteoinduction [27]. The platelet serves an important role as the carrier of abundant growth factors to direct wound healing. The increased surface area at bone and implant contact would be of considerable advantage if osseointegration represents a cohesive bond between the implant and bone. Surface topography also alters adherent cell production of significant cytokines and growth factors. The local release of these factors may then influence cells in the surgical microgap and at the surgical bone margin. Square thread form implant design have more surface area than reverse buttress thread form and PRP provides a pool of activated platelets, so this biomechanical combination can help in providing improved implant stability and enhance osseointegration. But the process of osseointegration is multifactorial and other factors should be considered like fixture design, surface characteristics, biocompatibility, state of host, biomechanical status, surgical techniques and Time.
Periotest is a non-invasive diagnostic method for evaluating implant–bone interface stability. IOPA x-rays are utilized in pre-surgical planning of implant treatment, intra-operatively, and for longitudinal assessment, especially for assessment of limited areas or individual implant sites. These have minimal distortion when they are well-angulated applying the standardized projection geometry and exposure dose is extremely low compared with that of other modalities [28].The unique features of the study included: a) It was a clinical study including human subjects; b) Implant placement was done in specified predetermined region of mandibular posterior area; c) Specific bone density i.e. D2 measured by CT scan was included; d) Implant angulation was determined with radiographic stent fabricated using milling machine; e) IOPA x-rays used to measure bone changes were standardized using putty occlusal jig; f) IOPA x-rays were obtained using paralleling technique with film holders which minimizes distortion; g) Single implant system and predetermined thread forms of implants were used throughout the study; h) Control group was included for each parameter.
Limitations of the study included: Short follow up period, Implant stability may be measured with more sensitive devices than Periotest and effect on bone was assessed in one dimension only i.e. height. However, this study has evaluated the effect of PRP and implant surface topography on implant stability and bone levels through three months follow up period, further studies are needed in controlled clinical trials before this approach can be used routinely. Moreover, further studies are needed to show whether the use of PRP during implant placement improves the prognosis of implants placed in different bone quality and contributes to shortening the healing time of dental implants. In summary, use of square thread form implants and local application of PRP is a relatively simple and convenient method and can be employed to enhance primary stability and bone healing.
* Mean, Standard Deviation and p-values- ‘two tailed’ for Periotest values (PTV) taken at baseline (Two weeks of implant placement), one month and three months of implant placement. †First versus second measurement. ‡Statistically significant (p-value <0.05)
*Mean, Standard Deviation and p-values- ‘two tailed’ for Bone levels on mesial side measured in mm taken at baseline (at time of implant placement), one month and three months of implant placement. †First versus second measurement. ‡Statistically non-significant (p-value >0.05)
*Mean, Standard Deviation and p-values- ‘two tailed’ for Bone levels on mesial side measured in mm taken at baseline (at time of implant placement), one month and three months of implant placement. †First versus second measurement. ‡Statistically non-significant (p-value >0.05)
*p-values- ‘two tailed’ for Periotest values and Bone levels on mesial & distal side measured in mm taken at baseline and one month of implant placement within same group. †p-values- ‘two tailed’ for Periotest values and Bone levels on mesial & distal side measured in mm taken at baseline and three months of implant placement within same group. ‡Statistically significant (p-value <0.05)