The microbial flora of the oral cavity is rich and extremely diverse. This reflects the abundant nutrients, moisture, hospitable temperature, and the availability of surfaces on which bacterial populations can develop. However, an imbalance in the microbial flora can lead to the production of acidic compounds by some microorganisms that can damage the teeth.
Development of the adherent populations of microorganisms in the oral cavity begins with the association and irreversible adhesion of certain bacteria in the tooth surface. This results in the formation of bacterial biofilms. Examples of some bacteria that are typically present as primary colonizers include Streptococcus, Actinomyces, Neisseria, and Veillonella. Examples of secondary colonizers include Fusobacterium nucleatum, Prevotella intermedia, and Capnocytophaga species [1].
Microorganisms are directly associated with the aetiology of enamel, dentin and pulpal pathology due to caries [2]. Secondary caries is the lesion at the margin of the existing restoration. These are called as “wall lesions” and they are results of microleakage.
Although the aetiologic factors of caries and the methods for its prevention have been widely investigated, secondary caries is the most common cause of dental restoration failure, presumably associated with residual bacteria and microleakage. Due to the high frequency of recurrent caries after restorative treatment, much attention has been paid to the therapeutic effects revealed by direct filling materials [3]. Besides the damage of the hard tissue, pulpal inflammation may be caused by invasion of the bacteria. Streptococcus species particularly Streptococcusmutans and Streptococcussorbinus, are associated with the initiation of the dental caries, and lactobacilli are associated with the progression of established lesion.
In spite of, considerable improvement in recent years, in composite restorations polymerization shrinkage and resultant contraction gaps at tooth-restoration interfaces continue to be a significant problem. Poor adaptation to the surrounding tooth substance may predispose to discoloration and bacterial colonization [4].
Secondary caries is the major factor that influences the longevity of dental restorations, particularly when composite restorations are placed with the cervical cavity margin in dentin [5]. This is the rationale for the use of disinfectants after cavity preparation and before placement of restoration, or including disinfectants or antibacterial agents directly into restorative materials.
A number of polymers with antibacterial properties were developed for this purpose, including soluble and insoluble pyridinium-type polymers involved in surface coating. Several reports have described incorporation of a methacryloyloxy dodecyl pyridinium bromide (MDPB) monomer in composite resins that showed no release of the incorporated monomer but still exhibited antibacterial properties. The antibacterial effects of composites for filling are mainly relevant to inhibition of plaque accumulation on the surface of the materials and tooth around the restoration. For dentin bonding systems, their antibacterial effects are relevant to disinfection of the cavity as well as inactivation of bacteria which invade the adhesive interface by microleakage [3].
This investigation was conducted in the laboratory to evaluate immediate and long term antibacterial properties of polymerized self-etching dentin adhesive systems.
Materials and Methods
Method of collection of data
Data was collected by measuring the two perpendicular diameters of the inhibition halo on a mitis salivarius agar plate in the ADT and recording the optical density, a measurement of turbidity that is based on the kinetics of bacterial growth, with the help of a spectrophotometer in the DCT.
Study materials
Four commercially available single bottle self etching dental adhesive systems used.
Adper Easy One 2) G bond 3) Clearfill S3 bond 4) Xeno V [Table/Fig-1].
Composition of the Bonding Agents
| Sl No. | Bonding Agent & Manufacterer | Composition |
|---|
| 1. | Adper Easy One (3M ESPE) | 2-hydroxyethyl methacryate (HEMA) Bis-GMA Methacrylated phosphoric esters 1,6 hexanediol dimethacrylate Methacrylate functionalized Polyalkenoic acid (Vitrebond” Copolymer) Finely dispersed bonded silica filler with 7 nm primary particle size Ethanol Water Initiators based on camphorquinone Stabilizers
|
| 2. | G Bond (GC Corporation) | Acetone 40% Distilled water 20% 4-Methacryloxyethyltrimellitateanhydride 15% Urethane dimethacrylate 10-20% Triethyleneglycol dimethacrylate 10%
|
| 3. | Clearfill S3 bond (Kuraray Medical Inc.) | 10-Methyacryloyloxydecyl dihydrogen phosphate.(MDP) Bis-phenol A diglycidylmethacrylate (Bis-GMA) 2-hydroxyethyl methacrylate (HEMA) Hydrophobic dimethacrylate dl-camphoroquinone ethyl alcohol water silanated colloidal silica
|
| 4. | Xeno V (Dentsply) | Bifunctional acrylic amides Acrylamido alkylsulfonic acid “inverse” functionalized phosphoric acid ester Acrylic acid Camphoroquinone Butylated benzenediol Water Tert-butanol
|
Instruments and equipments used are 96 well microtiter plates, Petri dishes, Mitis Salivaris Agar base, Micropipettes, Incubator, Phosphate buffered saline (PBS), Spectrophotometer, Light curing unit.
Test microorganism and Growth conditions
Streptococcus mutans 27351M, employed widely for testing antimicrobial activity of dental adhesive materials were obtained from the Department of Microbiology. Bacteria were grown aerobically from frozen stock cultures in brain heart infusion (BHI) broth at 37°C.
Agar Diffusion Test (ADT)
ADT was performed on mitis salivarius agar plates, in which four holes that were 4mm in diameter were punched in each plate with blunt end of sterile pasteur pipette tip with 4mm diameter. Then 200 microliters of freshly grown S.mutans spread evenly with a drigalsky glass stick. The four holes were immediately filled with 50 microliters of four test materials and light polymerized them using a light curing unit according to the manufacturer’s instructions.
In the first hole Adper easy one was filled, the second hole was filled with G-Bond, the third hole was filled with Clearfil S3 bond and the fourth hole was filled with Xeno V bonding agent. The agar plates were incubated for 72 hours at 37°C and then inspected for the presence of an inhibition zone. Two perpendicular measurements of the inhibition halo diameter were taken using a ruler.
Direct Contact Test (DCT)
The direct contact test is based on turbidometric determination of bacterial growth in 96-well microtiter plates [Table/Fig-2]. The kinetics of the outgrowth in each well is monitored at 630 nm at 37°C and recorded every 30min using a spectrophotometer.
Schematic representation of the DCT
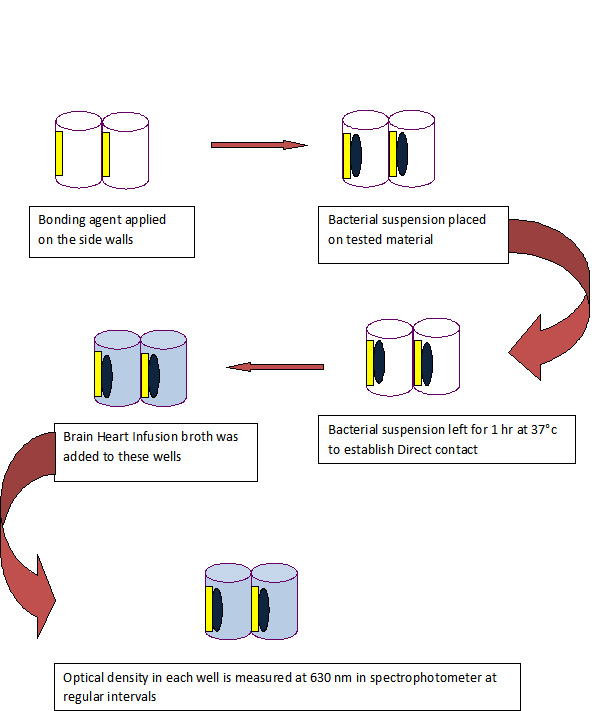
To create the samples, the side walls of the eight wells of 96 well microtitre plate of each tested material were evenly coated and polymerized using a light curing unit as per the manufacturer’s instructions.
For Adper Easy One, the bonding agent is applied only to the side walls for 20sec with an applicator and light cured for 10sec.
For G Bond, the bonding agent is applied only to the side walls, left for 5-10sec and light cured for 10sec.
For Clearfil S3 bond, the bonding agent is applied only to the side walls, left for 20 sec and light cured for 10sec.
For Xeno V, the bonding agent is applied only to the side walls twice, left for 20 seconds and light cured for 20sec.
All the tested materials were rinsed with phosphate buffered saline (PBS) before inoculating them with bacteria. 10 μL of bacterial suspension was placed on the tested material on the side walls of the wells. The plate was held in a vertical position and wells were inspected for evaporation of the suspension’s liquid, which occurred within 1h at 37°C. This ensured direct contact between bacteria and tested material. Brain Heart Infusion broth (220 μL) was added to each of these wells and gently mixed for 2min.
The positive control samples were eight wells with bacterial suspension only.
The microtiter plate was placed for incubation at 37°C in the incubator and the optical density in each well was measured at 630 nm in spectrophotometer. The readings were taken at regular intervals (Every 30min for 16h).
The antibacterial properties of the tested materials were examined using DCT immediately after polymerization and after aging them in PBS for one, two, seven and fourteen days. During the aging process, the PBS was renewed every 24h.The whole experiment was carried out under aseptic conditions and was repeated three times to ensure reproducibility.
Data were recorded, then plotted and statistically analyzed using ANOVA and Tukey’ test.
Results
The present study evaluated the antibacterial efficacy, immediate and long term antibacterial effects of Adper easy one, G-bond, Clearfil S3 bond and Xeno V with ADT and DCT.
In the ADT, inhibitory halos were found around all the bonding agents, with greater inhibition halo seen around Xeno V after incubating for 72h at 37°C [Table/Fig-3,4].
Inhibition halo diameters measured perpendicularly on 1st agar plate
| Bonding agent | Diameter 1 | Diameter 2 |
|---|
| Adper easy one | 21 mm | 20 mm |
| G Bond | 22 mm | 21 mm |
| Clearfil s3 Bond | 18 mm | 21 mm |
| Xeno V | 34 mm | 30 mm |
Inhibition halo diameters measured perpendicularly on 2nd agar plate
| Bonding agent | Diameter 1 | Diameter 2 |
|---|
| Adper easy one | 22 mm | 22 mm |
| G Bond | 24 mm | 22 mm |
| Clearfil s3 Bond | 21 mm | 20 mm |
| Xeno V | 30 mm | 34 mm |
The readings of the DCT were plotted on the graphs [Table/Fig-5,6,7,8and9].
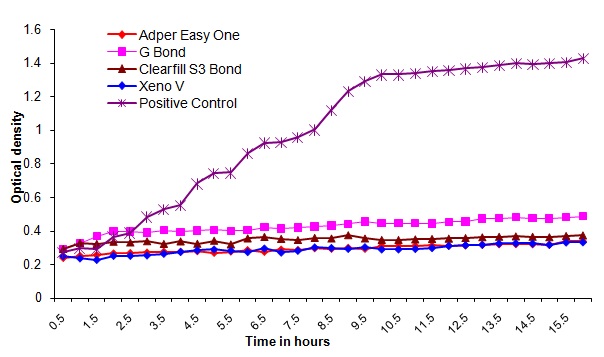
Bacterial outgrowth (measured by changes in optical density [OD]) on the surface of self-etching adhesive materials immediately after polymerization. Each point is the average OD measured simultaneously in eight wells for 16 hours
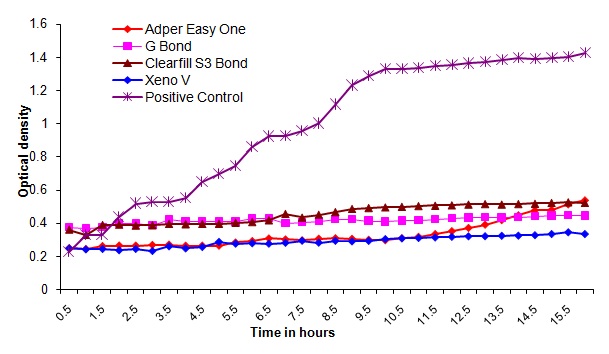
Bacterial outgrowth (measured by changes in optical density [OD]) on the surface of self-etching adhesive materials after aging for 1day. Each point is the average OD measured simultaneously in eight wells for 16 hours
Bacterial outgrowth (measured by changes in optical density [OD]) on the surface of self-etching adhesive materials after aging for 2 days. Each point is the average OD measured simultaneously in eight wells for 16 hours
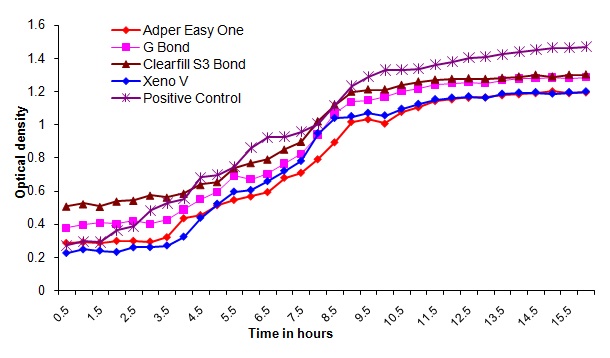
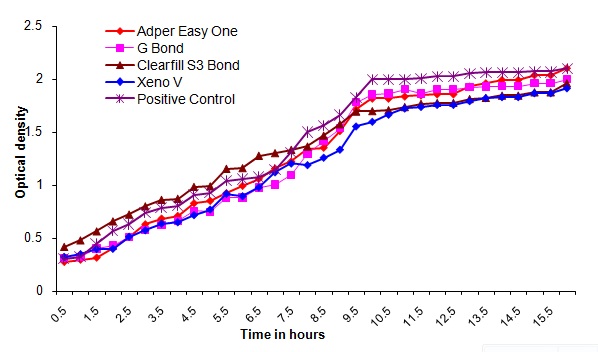
Bacterial outgrowth (measured by changes in optical density [OD]) on the surface of self-etching adhesive materials after aging for 7 days. Each point is the average OD measured simultaneously in eight wells for 16 hours
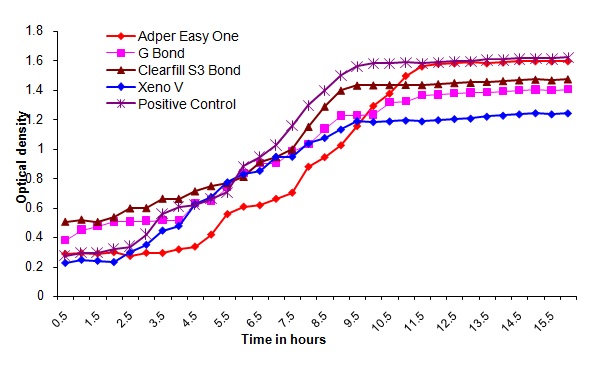
Bacterial outgrowth (measured by changes in optical density [OD]) on the surface of self-etching adhesive materials after aging for 14 days. Each point is the average OD measured simultaneously in eight wells for 16 hours
Discussion
Dental caries is an infectious disease of bacterial aetiology. Although several species of bacteria may be isolated from plaque associated with carious lesions, evidence suggests that Streptococcus mutans, Streptococcus sorbinus and lactobacilli are the major human dental pathogens [5]. Streptococcus mutans, harboured in dental biofilm, has been implicated as a primary causative agent for carious lesion. Its ability to synthesize extracellular glucans via glucosyltransferases (GTFs) is essential for sucrose dependent cellular adhesion and biofilm formation, and thus it is recognized as a critical virulence factor in the pathogenesis of dental caries [6]. Once the carious lesion is established, caries removal and an adequate cavity preparation are required as the part of the restorative treatment [7]. It is well recognised that residual bacteria after removal of a carious lesion cause increased pulpal sensitivity, pulpal inflammation and secondary caries [8].
Secondary caries is a major factor that influences the longevity of dental restorations [5]. The aetiology of secondary caries is primarily a mutans streptococci [9]. Due to high frequency of recurrent caries after restorative treatment, much attention has been paid to therapeutic effects revealed by direct filling materials. Composite restorations consist of two major components; a resin composite for filling and the dentin bonding systems (DBS) to be applied to the cavity before the placement of filling materials. Restorations must be sealed to prevent bacterial infection. Although dentin bonding systems have been developed to seal cavity preparations and prevent marginal leakage, even the latest bonding systems are not capable of providing a complete seal in clinical conditions [10]. Svanberg et al., proved that resin based materials tend to accumulate more dental biofilm than other restorative materials [11].
Brannstrom has reported that residual bacteria in the cavity preparation have been shown to multiply within the smear layer even in the presence of a good seal from the oral cavity [12].
Currently, polymerizable cationic monomers, which can covalently bound within the polymer matrix, can be introduced to provide resin-based dental materials with antibacterial activity. DBS with cationic monomers like methacryloyloxy dodecyl pyridinium bromide (MDPB), which is quaternary ammonium compound and methacryloxylethyl cetyl dimethyl ammonium chloride ( DMAE-CB) showed to inhibit biofilm accumulation on their surface [6]. Previous studies had reported that the reduction in the amount of bacteria at the tooth-restoration interface could be expected to influence the incidence of dental caries. Therefore, antibacterial activity is an important property of materials for successful restoration.
Dentine adhesives are currently available as three-step, two-step, and single-step systems depending on how the three cardinal steps of etching, priming, and bonding to the tooth substrate are accomplished or simplified. In the three-step system, etching, priming, and bonding are carried out in three different steps. Two-step systems are subdivided into self-priming adhesives that require a separate etching step, and self-etching primers that require an additional bonding step. The recently introduced, all-in-one adhesives have further combined these three bonding procedures into a single-step application [13].
Thus in the present study S.mutans was used to compare the antibacterial ability of single bottle self-etching dental bonding systems.
In self-etching adhesive systems, the pH of the self-etching primer solution is sufficiently low to demineralise the smear layer and the underlying dentin surface, so that etching and priming of the cavity can be accomplished simultaneously [14]. Due to the nonrinsing procedure, residual bacteria may remain at the interface between the tooth and the restorative material. The dentin primer is the component that comes into contact and reacts with the dentin substrate at the first stage of restoration in an adhesive system. If the tooth conditioners, such as primers, possessed antibacterial activity, these bacteria could be eliminated, thereby preventing secondary caries. Thus, the antibacterial activity of these adhesive systems that are directly applied to the dentin plays an important role in the longevity of the restoration [14].
The Direct Contact Test (DCT) has many advantages over agar diffusion test and has been studied previously by Weiss et al., Shalhav et al., and Fuss et al., [15]. It is a quantitative assay which allows water insoluble materials to be tested. In the present study, antibacterial activity of polymerized self-etching dental adhesive systems were examined for 16h after polymerization of the bonding agents because the logarithmic growth phase of a bacterial cell cycle ranges up to 14 to 16h.
The antibacterial activity of four self etching dental adhesive systems was evaluated in this study using ADT and DCT.
ADT was interpreted with inhibition halo as mark of the antibacterial property. The inhibitory halo’s are found around Adper easy one, G-bond, Clearfil S3 bond and Xeno V. The two perpendicular measurements of the inhibition halo diameters around the different bonding agents on mitis salivarious agar plate after incubation for 72h at 37°C are recorded. [Table/Fig-1,2]. Inhibition zones of 20-22 mm were present around Adper easy one, which was almost similar to the zones of inhibition around the G-bond and Clearfil S3 bond. Xeno V produced an inhibition zone ranging from 30-34mm, which was more than that produced by the other bonding systems.
The inhibitory zones formed around these bonding agents may be derived due to similarity of acidity of the bonding agents. Greater zone of inhibition around Xeno V may be attributed to its low pH (<2) compared to other bonding agents, (Adper easy one pH-3.5, Clearfil S3 bond pH-2.7 and G-Bond pH≈2) where their pH is greater than two. The bactericidal activities of self-etching primers elicited at a low pH are not reliable as they were ineffective against acid tolerant bacteria such as lactobacilli and Actinomyces. In addition, the acidity of self-etching primer is buffered by contact with dentin, and dilution of the solution by dentinal fluid reduces the antibacterial effects [3].Therefore the antibacterial effect of DBS due to their low pH should be considered to be limited. The light activation reduced significantly or suppressed the antibacterial activity of the initially active dentin bonding systems [16].The advantage of this method is that it allows direct comparisons of test materials against the test microorganisms, indicating which test materials have the potential to eliminate bacteria in the local micro environment. Further, the results of ADT can indicate the existence of diffusible components into an aqueous milieu [17].
Individual effect of each component present in the bonding agents and its antibacterial effect cannot be ruled out. The results from this test are confirmed by DCT in this study.
The direct contact test is based on turbidometric determination of bacterial growth in 96-well microtiter plates. The kinetics of the outgrowth in each well is monitored at 630 nm at 37°C and recorded every 30min using a spectrophotometer for 16h. Observations from this study showed that all the bonding agents exhibited similar optical density readings immediately after polymerization and after aging for one day in PBS. It was also observed that the optical density readings increased after aging for 2, 7 and 14 days in PBS. The optical density readings in the control group were high in all the measurements compared to the readings of the tested materials. Of all the bonding agents tested Xeno V performed better than other bonding agents( Adper easy one, G bond and Clearfil S3 bond) with statistical significance. (p<0.001) This can be attributed to the acidity, components of this bonding agent like acryloamido alkylsulfonic acid, “inverse” functionalized phosphoric acid ester, acrylic acid and tertiary butanol.
Antibacterial activity of adhesive systems depends upon several factors, including composition and acidity. Adhesion promoting monomers are considered to be one of the relevant elements of the antibacterial effect of the primers or adhesive solutions, since they confer acidic properties due to phosphoric, carboxylic or acrylic portions in the molecules [10]. In particular, self-etching bonding agents contain large amounts of acidic monomers in order to exert etching effect within 20 seconds. Individual effect of each component present in the bonding agents and its antibacterial effect are unknown. Further studies considering these aspects of bonding agents are required before conclusions are drawn.
Observations from this study showed that the antimicrobial activity of the self-etching dental adhesives during the time intervals was that, all the tested adhesives had an immediate bactericidal effect on Streptococcus mutans. However, all of the materials lost antibacterial properties within seven days. Adper easy one, G-Bond, clearfil S3 bond and Xeno V inhibited the bacterial growth immediately and upto 24h. It can be assumed that the antibacterial component decomposed at varying rates into the surrounding aging liquid. This is in agreement with the study conducted by Osnat Feuerstein et al., which concluded that self-etching dental adhesive systems exhibited immediate bactericidal effect on Streptococcus mutans [18]. The long term antibacterial activity of these materials remains to be evaluated.
All the bonding agents used in this study are single bottle self-etching dental adhesive systems. None of the bonding agents used in this study contained MDPB monomer which was proved to have long term antibacterial properties (upto 7 days) in many studies. The self-etching adhesive containing the MDPB molecule showed antibacterial activity for a week and could be recommended in situations where total disinfection of cavity is not accomplished due to lack of accessibility [13].
The results of our study are in agreement with the study done by Imazo et al., who suggested the benefit of low pH environment exhibited by dentine bonding agents as limited [19].
The results of this study showed short term antibacterial property of self-etching dentin bonding systems. Due to nonrinsing procedure, these self etching bonding systems can inactivate the residual bacteria retained in the cavity after cavity preparation. However, they may not be effective against bacteria which invade the tooth restoration interface due to microleakage which is observed clinically over a period of time.
Further invivo and invitro studies are necessary to determine the long term antibacterial effect of the single bottle self etching dentin bonding systems and to determine the benefit of dentin bonding systems with antibacterial properties for clinical applications. Furthermore, the depth of bacterial invasion into the dentinal tubules is another component to be investigated and also different conditions, such as salivary influence and biofilm formation should be investigated.
Conclusion
All the bonding agents showed antibacterial properties immediately and after one day after polymerization. It was observed in DCT that the antibacterial effect decreased by one week. Hence, it can be concluded that the four self-etching dental adhesive systems used in this study showed short term antibacterial property and none of these agents had long lasting effects. Additional studies both in vitro and in vivo are recommended to evaluate the long term antimicrobial effects of the self-etching dental adhesive systems.