Aims: To assess the effect of herbal antimicrobial agents on Streptococcus mutans count in biofilm formations during orthodontic treatment.
Materials and Methods: We calculated the growth inhibition of oral bacteria in the orthodontic appliances after herbal antibacterial agents were placed in culture media. The Minimum Inhibitory Concentrations (MICs) of these agents on Streptococcus mutans growth were determined. After cultivating colonies of Streptococci in biofilm medium with these herbal antimicrobial agents and orthodontic attachments, viable cell counting was performed from the bacteria which were attached on them. Scanning electron microscopy (SEM) analysis of morphology was observed on bacterial cells which were attached to orthodontic attachments. The effects of these agents were then evaluated and recommendations were forwarded.
Results: There was an increase in count of Streptococcus mutans with respect to the herbal antibacterial agents.
Conclusion: Despite the antibacterial functions of these herbal agents, there was increase in the biofilm formation caused by Streptococcus mutans to orthodontic bands, which had occurred most likely through upregulation of glucosyl transferase expression. These extracts may thus play an important role in increased bacterial attachment to orthodontic wires. Thus, this study was corroborative of an amalgamation of Ayurvedic therapy and Orthodontic treatment.
Introduction
The biofilm that forms on the surface of teeth, which is called dental plaque, has the ability to induce some of the most common diseases which afflict the oral cavity, including caries, gingivitis, and periodontitis [1]. Streptococcus mutans, which has been implicated as a primary aetiological agent of dental caries in animals and humans, plays an important role in plaque formation and accumulation [2]. The S. mutans properties, both acidogenic and aciduric, together with its ability to synthesize extracellular glucans, are considered the major factors for the development and establishment of cariogenic biofilms [2‚3]. Glucans, synthesized from dietary sucrose by glucosyl transferases (GTFs), enhances the pathogenic potential of dental plaque by promoting the adherence and accumulation of cariogenic Streptococci on the tooth surface, and by contributing to the bulk and structural integrity of plaque [4–6]. Streptococcus mutans produces at least three GTFs: B,C and D, which are critical for the adherence of bacteria to the surface of teeth and to each other. Orthodontic band placement which is done during the course of Orthodontic treatment tends to create new surfaces for the accumulation of plaque. It thereby increases the level of microorganisms in the oral cavity. There are elevations in levels of Streptococci and lactobacilli. In addition, orthodontic patients with fixed appliances frequently present an abundance of Streptococcus mutans in plaque as compared to untreated orthodontic patients [7]. Therefore, prevention of bacterial attachment to orthodontic wires and bands is a critical concern for orthodontists [8]. The three herbal agents are known to have antibacterial effects. Their effects on dental oral biofilms have not been well studied. As Streptococcus mutans exists almost exclusively in oral biofilms and it being the primary aetiologic agent of human dental caries,[9] we evaluated the effects of these three herbal agents on biofilm formations caused by Streptococcus mutans on orthodontic bands in vitro.
This study focused on the efficacies of these commonly used antibacterial agents against Streptococcus mutans, their counts around orthodontic molar bands, which was the commonest site for bacterial plaque accumulation. Viable cell counting and Scanning Dentistry SectionElectron Microscope studies were performed to assess the surfaces for microbial attachments.
Materials and Methods
We used 20 fresh stainless steel posterior bands of a liberal company to be cultured with the following test agents:
Test Agents
Three herbal antibacterial agents were used: Aswagandha, Triphala and Brahmi. Aswagandha (Withania somnifera), which is commonly referred to as Indian ginseng/ Winter cherry is locally applied to tumours, tubercular glands, carbuncles and ulcers [7]. It has been claimed to have a variety of health promoting effects which range from anti-inflammatory to anti-bacterial to immuno-modulatory to anti-stressor radio-sensitizer to antitumour. Triphala is a mixture of Amalaki (Emblica officinalis), Haritaki (Chebulic myrobalan) and Bhibitaki (Beleric myrobalan), which is used as gastrointestinal tract tonifier and an intestinal cleanser. Brahmi (Bacopa monniera) is used as a mental tonic and it rejuvenates the body. It is a promoter of memory and a nerve tonic.
Bacterial growth and herbal extracts procedure: Effects of three different plant extracts on bacterial biofilm formations on orthodontic bands. Steptococcus mutans was maintained on brain heart infusion (BHI) agar medium and grown under aerobic conditions.The biofilm assay was performed in biofilm medium (BM) which contained 3% sucrose. The three plant extract materials were formulated in DMSO and taken into different concentrations as per the requirements.
Growth inhibition produced by well-diffusion method
Steptococcus mutans was inoculated in BHI broth and incubated for 4–6 hours, to the point when growth was considered to be in the logarithmic phase. The density of the bacterial suspension was adjusted with sterile phosphate buffer saline (PBS) to match the density of McFarland’s standard 0.5. The bacterial broth suspension was streaked evenly onto the BHI agar plates with a cotton swab. After the inoculum had dried, an 8-mm wells were made using a cork borer, 50ul of the plant extracts was added and the plate was kept incubated at 37oC in an aerobic condition. Diameters of the zones were measured at three different points.
Minimum inhibitory concentration determination
Minimum Inhibitory Concentration Determination was done by using tube dilution method (MIC) and the three plant extracts (material), where their concentrations were tested in triplicate at serial dilutions of 0, 1, 2, 4, 8, 16, 32, 64, and 128 mg/ml. Each test tube was filled with 15 ml BHI broth which contained the three plant extracts at different concentrations, which was prepared earlier with 1ml of inoculated broth and incubated overnight at 37oC. To establish the specific MICs, turbidometric measurements were carried out to identify the lowest concentrations of the materials, which inhibited the growth and the turbidity.
Bacterial attachment on orthodontic wire
To evaluate the effects of the three different plant extracts on bacterial biofilm formations, we used sterile orthodontic wire for biofilm formation. S. mutans was cultured in a test tube which contained 15 ml of BM-sucrose broth which was soaked with 2-cm wire and sub-MIC of the three extracts after 40- hour incubation at 37oC under aerobic condition, each wire was washed twice in sterile PBS (pH 7.2) and moved to another sterile test tube. For viable cell counting, each band was incubated with Streptococci and the three plant extracts in BM-sucrose for 40 hours and was sonicated in 1mL of PBS. The PBS was serially diluted to 1/10,000 and each 100ul was spread on BHI agar plate. After incubation for 2– 3 days, bacterial colonies from each plate were counted and the relative colony-forming units (CFUs) were calculated.
Scanning electron microscope (SEM)
SEM studies were performed to evaluate the surfaces which were treated with the herbal agents.
Results
Our study showed significant results [Table/Fig-1] shows the various biochemical tests which were carried out for the samples which were isolated from the dental swabs. The organism which was detected showed Gram positivity and lactose fermentation, thus suggesting that it was Streptococcus mutans, which was the most important bacterium among those which were involved in early colonization in plaque formation. Growth Inhibition Zones formed against Streptococcus mutans by the three herbal agents were observed, as shown in [Table/Fig-2]. The MICs of herbal extracts against Streptococcus mutans were determined by using tube dilution method. Erythromycin was considered as the standard drug. It showed the lowest MIC (10 μg/mL). MIC against Triphala was 32-42 mg/mL and those against Aswagandha and Brahmi were greater than 128 mg/ mL [Table/Fig-3]. On measuring the effect of garlic on bacterial attachment to orthodontic band, we interestingly found that the viable cell count [Table/Fig-4] increased continuously, suggesting that more bacterial cells had attached to the orthodontic band in the presence of triphala than in the presence of Aswagandha and Brahmi. The figure shows SEM images of Streptococcus mutans on orthodontic band. As compared to those in control, bacterial attachment and aggregation on band had notably increased in the herbal agents [Table/Fig-5].
Biochemical tests for the sample isolated from the dental swab
| S.No. | Test | Result |
| 1. | Gram staining | Gram positive cocci in chains |
| 2. | On Mac.Conkey agar | Lactose fermentation observed |
| 3. | Sugar fermentations ( TSI) | Ferments – lactose, glucose, sucrose |
| 4. | Catalase | negative |
Growth inhibition zones against oral bacteria by the three herbal agents
| Plant Name | Zones of inhibition in mm |
| Erthromycin | 32mm |
| Triphala | 10mm |
| Aswagandha | nil |
| Brhami churna | nil |
Minimum inhibitory concentrations of the herbal agents against Streptococcus mutans
| Test Agent. | Cell Count |
| Erthromycin | › 0.03 x 105 CFU/ml |
| Triphala | › 0.55 x 105 CFU/ml |
| Aswagandha | 0.55 x 105 CFU/ml |
| Brhami | 0.35 x 105 CFU/ml |
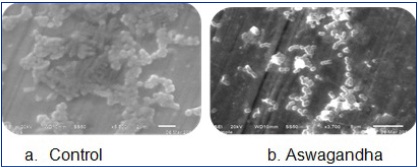
SEM images of the three herbal agents

Discussion
The physical removal of the early Streptococcal rich biofilm, as was practised at least as early as in ancient Egyptian civilization, has attained a solid scientific foundation [10].
Herbal agents have wide spectra of antibacterial activities. In this study, we tried to uncover the effects of herbal agents on dental biofilm formations by using S. mutans, by analyzing attachment on orthodontic band. Due to their known antibiotic functions, we hypothesized that they would most likely inhibit bacterial attachment to orthodontic bands via their antibacterial effects. Against expectation, however, these agents actually increased bacterial biofilm formations. In agreement with viable counting of the bacterial cells on the bands, SEM image showed clear effects of these agents, especially Triphala, on S. mutans growth in terms of increased attachment. This was in contrast to findings of a study, where it showed more potency on E. faecalis biofilm. This may be attributed to its formulation, which contains three different medicinal plants in equal proportions. Tannic acid represents the major constituent of the ripe fruits of T .chebula, T.belerica and E. officinalis. Earlier studies have reported that tannic acid was bacteriostatic or bactericidal for some gram positive and gram negative pathogens [11]. A possible explanation could be that these extracts actually contained a biologically active substance which was effective at low doses, for gene activation, prior to bacterial cell growth inhibition.
Bacterial attachment is the initial step in the formation of biofilm communities. Streptococcus mutans proliferates as a biofilm on tooth surfaces, where it obtains nutrients and metabolizes fermentable dietary carbohydrates [12]. The GTFs, in concert with glucan-binding proteins, contribute greatly to initial attachment and to the formation of biofilms. S epidermidis biofilm formation is known to be associated with the production of the polysaccharide intercellular adhesin (PIA) and poly-Nacetylglucosamine polysaccharide (PNAG). Recently obtained evidence has indicated that Staphylococcal accessory regulator (SarA), a central regulatory element that controls the production of S aureus virulence factors, is essential for the synthesis of PIA/PNAG and that it ensues biofilm development in this species [13]. These results suggested that the enzymes which participated in bacterial biofilmformations were specific for bacteria, and that the increase in bacterial attachments caused by these agents occurred through upregulation of GTF family genes. Their expressions were therefore very specific for S. mutans [14].
Previous research done on the regulatory mechanisms of GTF family genes in S. mutans showed that biofilm acidifications or excess metabolizable carbohydrates (glucose or sucrose) could induce GTF gene expression [15]. Furthermore, the structure ofpolysaccharide matrix changes over time, as a result of the action of mutanases and dextranases which are present within plaque. GTFs, at distinct loci, offer chemotherapeutic targets to prevent caries. Nevertheless, agents that inhibit GTFs in solution, frequently have reduced or no effects on adsorbed enzymes. Clearly, conformational changes and reactions of GTFs on surfaces are complex and they modulate the pathogenesis of dental caries in situ [16]. Hence, strategies which target an S. mutans density dependent quorum sensing system to attenuate biofilm formation and/or virulence, are currently being used, to develop therapeutic or preventive measures against dental caries [17]. Catabolite Control Protein A (CcpA) has a major role in Carbon Catabolite Repression (CCR) and regulation of gene expression, but it has been revealed that in S. mutans, there was a substantial CcpA-independent network that regulated gene expression in response to the carbohydrate source. Based on genetic studies, biochemical and physiological experiments have demonstrated that loss of CcpA impacted the ability of S. mutans in transporting and growing on selected sugars [18].
The development of novel technologies and the rapid advances made in dissecting the genetics and physiology of S. mutans, have resulted in the emergence of this bacterium as a new Gram positive model organism and they have proved that S. mutans was an amyloid-forming organism that contributed to biofilm formations [19]. This study may provide an impetus for understanding the interrelations between the plaque biofilm, tooth tissues and the oral environment, and for the development of procedures to modify the course of caries development [20]. Thus, the progress made in recent decades places S. mutans in an interesting position, to further make advances in basic microbiology research done especially on Gram-positive organisms [21].
Conclusion
Herbal agents increase bacterial biofilm formations on orthodontic band in a concentration-dependent manner. These agents seem to contain biological materials that promote formation of biofilms via activation of Glucosyl Transferases (GTFs). The present findings may offer fresh insight into herbal agents which induced GTF expression in S. mutans at the molecular level and into potential consequences which could occur, so that proper care of orthodontic bands could be undertaken.
Limitations
In order to verify the changes which occurred in Glucosyltransferase (GTF) expression levels under the effects of these herbal agents, real-time PCR needs to be performed. Other methods like ATP luminescence assay can also be used for easily detecting bacteria.
[1]. MA Taubman, DA Nash, The scientific and public-health imperative for a vaccine against dental caries. Great Britain: The Caister Academic Press; 2006. [Google Scholar]
[2]. WJ Loesche, Role of Streptococcus mutans in human dental decay.Microbiology Reviews. 1986 50(4):353-80. [Google Scholar]
[3]. PD Marsh, DJ Bradshaw, Dental plaque as a biofilm.Journal of Industrial Microbiology. 1995 15(3):169-75. [Google Scholar]
[4]. P Marsh, B Guggenheim, R Schmid, Caries Res.Journal of Industrial Microbiology. 1972 6(2):103-21. [Google Scholar]
[5]. KM Schilling, WH Bowen, Glucans synthesized in situ in experimental salivary pellicle function as specific binding sites for Streptococcus mutans.Infection and Immunity. 1993 60(1):284-95. [Google Scholar]
[6]. JM Tanzer, ML Freedman, RJ Fitzgerald, Virulence of mutants defective in glucosyltransferase, dextran-mediated aggregation, or dextranase activity. In: Mergenhagen SE and Rosan B, editors.Molecular Basis of Oral Microbial Adhesion.Washington Press;1985 [Google Scholar]
[7]. JA Corbett, LR Brown, HJ Keene, IM Horton, Comparison of concentrations in non-banded and banded orthodontic patientsJ Dent Res. 1981 60(12):1936-42. [Google Scholar]
[8]. JW Balenseifen, JV Madonia, RJ Fitzgerald, Study of dental plaque in orthodontic patients.J Dent Res. 1970 49(2):320-4. [Google Scholar]
[9]. TJ Mitchell, The pathogenesis of streptococcal infections: from tooth decay to meningitis.Nat Rev Microbiol. 2003 1:219-30. [Google Scholar]
[10]. Whitmore Sarah E, Lamont Richard J, The pathogenic persona of community associated oral streptococci.Mol. Microbiol. 2011 81(2):305-14. [Google Scholar]
[11]. Pujar Madhu, Patil Chetan, Kadam Ajay, Comparison of antimicrobial efficacy of Triphala, (GTP) Green tea polyphenols and 3% of sodium hypochlorite on Enterococcus faecalis biofilms formed on tooth substrate: in vitro.J. Int Oral Health. 2011 3(2):23-30. [Google Scholar]
[12]. EG Smith, GA Spatafora, Gene regulation in Streptococcus mutans: complex control in a complex environment:J Dent Res. 2012 91(2):133-41. [Google Scholar]
[13]. MA Tormo, M Marti, J Valle, AC Manna, I Lasa, al et, SarA is an essential positive regulator of Staphylococcus epidermidis biofilm development.J Bacteriol. 2005 187(7):2348-56. [Google Scholar]
[14]. Lee HeonJin, Park Hyo–Sang, Kim Kyo–Han, Kwon Tae–Yub, Hong Su–Hyung, Effect of garlic on bacterial biofilm formation on orthodontic wire.Angle Orthod 2011 81(5):895-900. [Google Scholar]
[15]. Y Li, RA Burne, Regulation of the gtfBC and ftf genes of Streptococcus mutans in biofilms in response to pH and carbohydrate.Microbiology. 2001 147:2841-8. [Google Scholar]
[16]. WH Bowen, H Koo, Biology of Streptococcus mutans-derived glucosyltransferases: role in extracellular matrix formation of cariogenic biofilms. Caries Res. 2011 45(1):69-86. [Google Scholar]
[17]. D Senadheera, DG Cvitkovitch, RJ Fitzgerald, Virulence of mutants defective in glucosyltransferase, dextran-mediated aggregation, or dextranase activity. In: Mergenhagen SE and Rosan B, editors.Adv Exp Med Biol. 2008 45:178-88. [Google Scholar]
[18]. J Abranches, MM Nascimento, L Browngardt, CM Wen, MF Rivera, al et, CcpA regulates central metabolism and virulence gene expression in Streptococcus mutans.J Bacteriol. 2008 190(7):2340-49. [Google Scholar]
[19]. MW Oli, HN Otoo, PJ Crowley, KP Heim, MM Nascimento, CB Ramsook, al et, Functional amyloid formation by Streptococcus mutans.Microbiology. 2012 158:2903-16. [Google Scholar]
[20]. M Shu, L Wong, JH Miller, CH Sissons, Development of multi-species consortia biofilms of oral bacteria as an enamel and root caries model system. Arch Oral Biol. 2000 45(1):27-40. [Google Scholar]
[21]. Lemos Jose´ A, Quivey Robert G, Jr Koo Hyun, Abranches Jacqueline, Streptococcus mutans: a new Gram-positive paradigm?. Microbiology. 2013 159(1):436-45. [Google Scholar]