Correlation of Plasma Lipid Profile with Salivary Oxidative Stress Markers in Type II Diabetes Mellitus Patients
Rajeshwari S G1, Afreen Arshad Choudhry2, Arpitha Gururaja3, Krishnananda Prabhu4
1 Postgraduate Student, Departmant of Biochemistry, Kasturba Medical College- Manipal, Manipal University, Manipal, India.
2 Postgraduate Student, Departmant of Biochemistry, Kasturba Medical College- Manipal, Manipal University, Manipal, India.
3 Postgraduate Student, Departmant of Biochemistry, Kasturba Medical College- Manipal, Manipal University, Manipal, India.
4 Associate Professor, Departmant of Biochemistry, Kasturba Medical College- Manipal, Manipal University, Manipal, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr Krishnananda Prabhu, Departmant of Biochemistry, Kasturba Medical College- Manipal, Manipal University, Manipal-576104, India.
Phone: 0820 2922326,
E-mail: Krishnakunj2000@yahoo.com
Introduction: Diabetes is known to be associated with greater production of reactive oxygen species (ROS) like malondialdehyde (MDA) and decreased antioxidants like total thiols and its estimation in plasma is used in monitoring the redox status. The aim of this study was to analyse the association between plasma lipid profile parameters like HDL and LDL with salivary MDA and thiols in diabetic patients.
Materials and Methods: Sixty subjects between the age group 35- 70 years who were diagnosed with type II diabetes mellitus based on fasting blood glucose and glycated haemoglobin levels who attended the dental OPD at Kasturba Medical College and Hospital, Manipal consented to participate in this study. Plasma glucose, HDL and LDL were estimated in Cobas autoanalyser by hexokinase method, homogenous enzymatic colorimetric assay and Friedwald’s formula respectively. Assessment of glycated hemoglobin was by ion exchange chromatography, MDA by thiobarbituric acid as a substrate and thiols by Ellmann’s manual method in plasma and saliva.
Results: The association of plasma LDL with salivary MDA was found to be positive and significant and that with salivary thiols was negative and significant. Also, the association of plasma HDL with salivary MDA was found to be negative and significant and that with salivary thiols was positive and significant.
Conclusion: Results indicate the potential of saliva as a tool to monitor prognosis of diabetes.
Dyslipidemia, Malondialdehyde, Saliva, Total thiols
Introduction
In recent years, there has been a tremendous rise in the prevalence of type II diabetes mellitus patients [1–3]. Dyslipidemia and oxidative stress has been implicated in pathogenesis of diabetes and associated complications like atherosclerosis and cardiovascular disease [4]. Diabetes is known to be associated with increased oxidative stress which is due to greater production of reactive oxygen species (ROS) and an impaired antioxidant defense mechanism. Malondialdehyde (MDA) is a product of lipid peroxidation and its estimation in plasma is widely used in monitoring the status of oxidative stress. In a similar fashion, protein thiols (-SH) constitute a major portion of total antioxidants in the body and hence it plays a major role in defense against ROS. The formation of free radicals beyond the scavenging ability of endogenous antioxidants in diabetes leads to a decrease in total antioxidant status of the body. Saliva is a biological fluid secreted abundantly and is a known ultra filtrate of blood and its components may be in proportion to their respective concentrations in plasma [5]. Moreover, the collection of saliva is easy and non invasive. The assessment of oxidants and antioxidants in saliva therefore may be used to measure the redox status of the body.
The aim of the present study is to analyse the association between plasma lipid HDL and LDL with salivary MDA and total thiols in diabetic patients.
Materials and Methods
Institutional Ethics Committee gave permission to carry out this study. This study was carried out from December 2012 to October 2013. The eligible participants during this study period were included in the study. Sixty subjects between the age group 35-70 years who were diagnosed with type II diabetes mellitus based on fasting blood glucose and glycated haemoglobin levels who attended the dental OPD at Kasturba Medical College and Hospital, Manipal consented to participate in this study.
Patients diagnosed with type I diabetes, other systemic diseases requiring long term medications, those with severe diabetic complications, with regular alcohol and tobacco habits and with salivary gland disorders were excluded from the study. Unstimulated saliva (5 ml) was collected by standard procedures in a sterile container. Two ml of blood was collected each in fluoride and plain vacutaiers for estimations of glucose and lipid profile respectively. Also, 2 ml sample for plasma MDA and total protein thiols estimations was collected in plain vacutainers. Blood glucose levels, HDL and LDL were estimated in Cobas 6000e autoanalyser by hexokinase method, homogenous enzymatic colorimetric assay and Friedwald’s formula respectively. Glycated hemoglobin was estimated by ion exchange chromatography using HPLC Variant turbo II. MDA was assessed by using thiobarbituric acid as a substrate and Thiol was estimated by Ellmann’s manual method in saliva. Data were analysed using Spearman’s correlation method by Statistical Package for the Social Sciences (SPSS 16).
Results
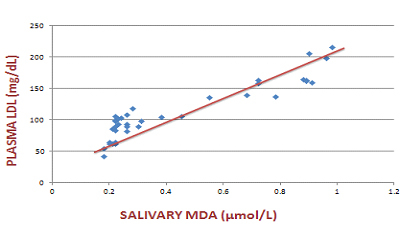
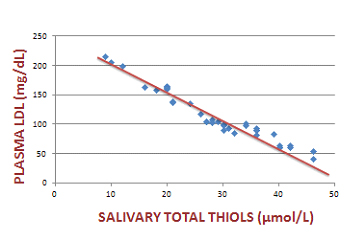
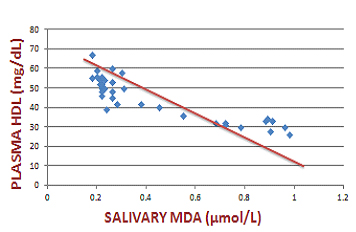
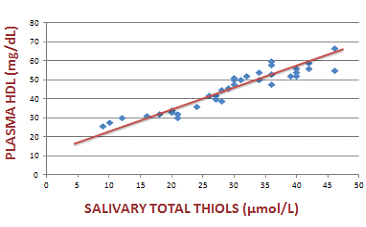
We compared plasma lipid profile with salivary oxidative stress markers using Spearman’s correlation coefficient. Plasma LDL showed a significant positive correlation with salivary MDA and a significant negative association with salivary total thiols. Similarly, plasma HDL showed a significant positive correlation with salivary total thiols and a significant negative association with salivary MDA levels. [Table/Fig-1,2,3,4and5] shows the association of plasma LDL and plasma HDL with salivary MDA and salivary total thiols.
Correlation of plasma HDL and LDL with salivary MDA and total thiols in the diabetic group
| PARAMETERS (in diabetic group) | rs VALUE (Spearman's Correlation coefficient) | p-VALUE |
|---|
| Plasma LDL and salivary MDA | 0.745 | <0.001 |
| Plasma LDL and salivary total thiols | -0.97 | <0.001 |
| Plasma HDL and salivary MDA | -0.84 | <0.001 |
| Plasma HDL and salivary total thiols | 0.94 | <0.001 |
Scatter plot that indicates the association of plasma LDL with salivary MDA in the diabetic group
Scatter plot that indicates the association of plasma LDL with salivary total thiols in the diabetic group
Scatter plot that indicates the association of plasma HDL with salivary MDA in the diabetic group
Scatter plot that indicates the association of plasma HDL with salivary total thiols in the diabetic group
Discussion
We compared plasma HDL and LDL with salivary oxidative markers to evaluate a possible role for saliva for screening disorders like Diabetes.
In the present study, a significant positive correlation was found between plasma LDL and HDL with salivary MDA and total thiols respectively. Also, a significant negative correlation was found between plasma LDL and HDL with salivary total thiols and MDA respectively. It has already been proved that long term diabetes is associated with a state of dyslipidemia [6]. The manifestation of dyslipidemia can be varied but is usually associated with decreased HDL and increased LDL as shown by our study. The hyperglycaemic state in diabetes mellitus itself contributes to increased oxidative stress in the body [7]. Dyslipidemia along with increased oxidative stress makes the individual more susceptible towards cardiovascular diseases like atherosclerosis and coronary heart diseases.
Oxidative status in individuals is evaluated using markers such as plasma malondialdehyde (MDA) which is a product of lipid peroxidation and is known to increase in dyslipidemia associated with diabetes mellitus and total protein thiols (-SH) which constitute a major portion of total antioxidants in the body and hence it plays a major role in defense against ROS which decreases in diabetes mellitus [8].
The prospect of choosing saliva as the biological fluid which can reflect the redox status is because it is a known ultra filtrate of blood and blood components which can be transferred across the salivary gland epithelium in proportion to their respective concentration in plasma [9]. The present study also supports this hypothesis as the results indicate a strong correlation between plasma and salivary markers. Another important aspect is that the collection of saliva is easy, non invasive and hence not traumatic for the patient. Saliva till recent years was known to have a role in physiological process like digestion due to the presence of digestive enzymes and maintenance of oral health. However, recent studies have shown that it may have a diagnostic importance [10].
As seen in the present study increased plasma LDL and decreased plasma HDL is associated with a simultaneous decrease of salivary total protein thiols and an increase in the salivary MDA levels. Antonio et al., Maharajan et al., and Mooradian show that diabetes associated dyslipidemia can cause endothelial dysfunction due to increased oxidative stress [11–13]. This result reflects the overwhelming response of the body towards oxidative stress which is reflected in the saliva also.
To conclude, results obtained by comparison of plasma lipid profile with the salivary oxidative stress markers show the potential for saliva as a non-invasive medium in screening and in monitoring prognosis of diabetes. This implicates a possibility for using saliva in screening and monitoring of various pathological conditions.
[1]. Ja YJ, Seung HK, Hyuk-Sang K, Nan HK, Jae HK, Chul SK, Prevalence of Diabetes and Prediabetes according to Fasting Plasma Glucose and HbA1cDiabetes Metab J 2013 37:349-57. [Google Scholar]
[2]. Choi SH, Kim TH, Lim S, Park KS, Jang HC, Cho NH, Hemoglobin A1c as a diagnostic tool for diabetes screening and new-onset diabetes prediction: a 6-year community-based prospective studyDiabetes Care 2011 34:944-9. [Google Scholar]
[3]. Bae JC, Rhee EJ, Lee WY, Park SE, Park CY, Oh KW, Optimal range of HbA1c for the prediction of future diabetes: a 4-year longitudinal studyDiabetes Res Clin Pract 2011 93:255-9. [Google Scholar]
[4]. Yonas M, Rajinder C, Tedla K, Yesehak W, Dyslipidemia Associated with Poor Glycemic Control in Type 2 Diabetes Mellitus and the Protective Effect of Metformin SupplementationInd J Clin Biochem 2012 27:363-9. [Google Scholar]
[5]. Natheer H Al-Rawi, Oxidative stress, antioxidant status and lipid profile in the saliva of type 2 diabeticsDiabetes & Vascular Disease Research 2011 8:22-8. [Google Scholar]
[6]. Betteridge DJ, Diabetic dyslipidaemiaEur J Clin Invest 1999 29:12-6. [Google Scholar]
[7]. American Diabetes AssociationDiagnosis and classification of diabetes mellitusDiabetes Care 2010 33(1):S62-9. [Google Scholar]
[8]. Shermin K, Pratibha PK, Vasudev G, Superoxide dismutase enzyme and thiol antioxidants in gingival crevicular fluid and salivaDent Res J 2012 9:266-72. [Google Scholar]
[9]. Reznick AZ, Shehadeh N, Shafir Y, Naglar RM, Free radicals related effects and antioxidants in saliva and serum of adolescents with type 1 diabetes mellitusArch Oral Biol 2006 51:640-8. [Google Scholar]
[10]. Arati S. P, Correlation of Salivary Glucose Level with Blood Glucose Level in Diabetes MellitusJ Oral Maxillofac Res 2012 3:1-7. [Google Scholar]
[11]. Ceriello A, Kumar S, Piconi L, Esposito K, Giugliano D, Simultaneous control of hyperglycemia and oxidative stress normalizes endothelial function in type 1 diabetesDiabetes Care 2007 30:649-54. [Google Scholar]
[12]. Mooradian AD, Dyslipidemia in type 2 diabetes mellitusNat Clin Pract Endocrinol Metab 2009 5:150-9. [Google Scholar]
[13]. Maharjan BR, Jha JC, Adhikeri D, Wishwanath P, Baxi J, Alurkar VM, A study of oxidative stress, antioxidant status and lipid profile in diabetic patient in Western region of NepalKathmandu Univ Med J 2008 6:16-21. [Google Scholar]