Alteration of Interferon Gamma (IFN-γ) in Human Plasma with Graded Physical Activity
Ambarish Vijayaraghava1, Radhika K2
1 Associate Professor, Department of Physiology, M.S. Ramaiah Medical College, Bangalore, India.
2 Lecturer Cum Statistician, Department of Preventive and Social Medicine, M.S. Ramaiah Medical College, Bangalore, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Ambarish Vijayaraghava, Associate Professor, Department of Physiology, M.S. Ramaiah Medical College, Mattikere, MSRIT Post, Bangalore-560054, India.
Phone: 09886475610,
E-mail: ambarishv@rediffmail.com, ambarish.vijayaraghava@gmail.com
Introduction: Practice of regular exercise is beneficial for health. Physical exercises have been demonstrated to alter levels of the cytokine interferon Gamma in plasma. IFN-γ is known to be an anti-inflammatory cytokine.
Materials and Methods: We assessed the effect of single bout of moderate exercise and a single bout of strenuous exercise and one month of regular moderate exercise on plasma IFN-γ. The study consisted of 18 healthy volunteers (10 males and 8 females) with the mean age, 20.94 years, range, 18-25 years. The exercise regime adopted is the standardized 10m Shuttle Walk Test regime. IFN-γ was estimated using the Sandwich ELISA technique. The reagent kit was obtained from Duoset ELISA Development System of R & D Systems Europe Ltd. The readings were taken at 450nm using Organon Teknika Reader 230S. Statistical methods: Friedman test has been used for analysing IFN-γ values.
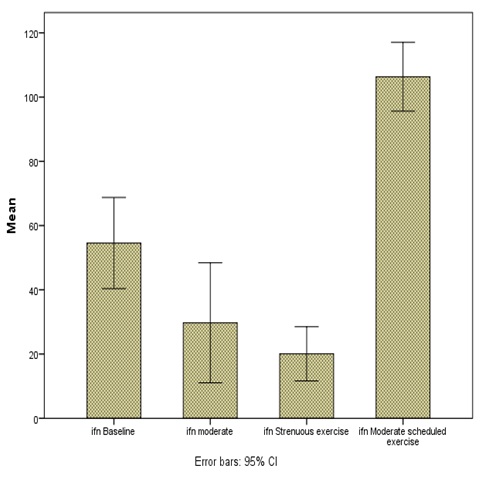
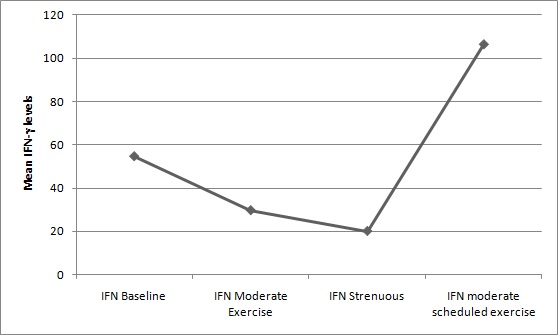
Results: Mean and SD values of IFN-γ (in picograms per ml) for baseline (no exercise) was: 54.56 ± 28.54 (log transformation: 1.68±0.23), for acute moderate exercise: 28.94 ± 38.46 (log transformation: 1.34 ± 0.24), for acute strenuous exercise: 20.06 ± 16.96 (log transformation: 1.18 ±0.33) and after one month of regular moderate exercise: 106.33 ± 21.51 (log transformation: 2.02 ± 0.09). The change in IFN-γ levels showed significant difference between; a) baseline and moderate exercise, b) baseline and strenuous exercise, c) moderate and strenuous exercise, d) strenuous exercise and end of one month of regular moderate exercise, e) baseline and end of one month of regular moderate exercise, f) moderate exercise and end of one month of regular moderate exercise. IFN-γ showed overall significance between different grades of exercise (p < 0.05).
Conclusion: Plasma IFN-γ decreases with one bout of acute moderate exercise, it decreases further with one bout of acute strenuous exercise and increases at end of one month of regular moderate exercise, which is more than baseline value. This shows that regular moderate exercise has beneficial effects on health by way of increasing plasma IFN-γ level.
Interferon gamma, Exercise, Inflammation
Introduction
The cytokine interferon-Gamma (IFN-γ) is considered as an anti-inflammatory cytokine in low concentrations [1]. Frequent and small bursts of inflammatory cytokine response especially of TNF-γ and IL-6, has been implicated for the genesis of several life style diseases [2]. Short bouts of unaccustomed exercises have been demonstrated to increase the serum IL-6 levels and the hsCRP (highly sensitive C reactive protein) to correlate with increased role for coronary artery disease and other cardiovascular diseases [3]. Persisting physical stress has been demonstrated to increase secretion of IL-6 leading to premature onset of lifestyle diseases [4]. The exercises of moderate nature has been shown to reduce the severity of inflammation in rheumatoid arthritis [5]. Daily practice of moderate exercise improves the performance of immune system in all age groups [6]. It is an established fact that regular moderate exercise improves the overall general health [7].
During the last two decades several studies have been done to see the changes in cytokine levels with different grades of exercises. There have been studies on subjects undertaking marathon, military training, downhill running on a treadmill, cycling, etc., on different groups of individuals in different parts of the world [8–11]. We undertook this study in order to understand the impact of moderate and strenuous exercise on the plasma levels of IFN-γ in unaccustomed individuals and the benefit of exercise on accustumisation by the same individuals.
Materials and Methods
Eighteen healthy volunteers, 10 males and 8 females in the age group of 18 to 25 years, with mean BMI of 21.65±1.84 (kg/m2) not performing any kind of regular exercise were chosen for this study. Sample size was calculated based on a previous study [12]. With an effect size of 0.66, power of 80 percentage and significance of 5 percentage, the minimum sample size was estimated to be 15. Prior consent was taken from all of them before inducting them into the study. Clearance was obtained from the ethical committee of the institution for the study.
All subjects were made to do one bout of moderate exercise (acute moderate exercise), one bout of strenuous exercise (acute strenuous exercise) and one month of scheduled moderate exercise on a daily basis. The subjects were made to perform acute moderate exercise on the first day and acute strenuous exercise on the second day. They were made to perform scheduled regular moderate exercise from the third day onwards, for 30 days with strict monitoring. During one month of scheduled moderate exercise, the subjects were made to perform single bout of moderate exercise daily for 30 days. The exercise was graded as moderate or strenuous based on the rise in heart rate. It was labelled as moderate when the heart rate increased by 50% from resting level and was labelled as strenuous when heart rate doubled [13].
Shuttle Walk Test Protocol: The exercise regime chosen was the standardized 10m Shuttle Walking test regime, described by Glenfield Hospital, Leicester, United Kingdom in collaboration with the department of Physical Education and Sports Science, Loughborough University of Technology, United Kingdom [14–17]. In this exercise protocol, the subjects walk on a 10 meter plain path at the two ends of which are placed marker cones. The subjects walk between the cones corresponding to the beeps given out by a record player. Subjects have to increase their speed of walking gradually in tandem with the shortening of intervals between the consecutive beeps as time progresses. The level of the shuttle walk regime at which the heart rate increased by 50% of the baseline was chosen as moderate exercise. The level at which the heart rate increased by 100%, i.e. doubled was considered as strenuous exercise.
A venous blood sample from cubital vein (using vacutainers) just before acute moderate exercise (baseline) was collected. Another sample was collected immediately after acute moderate exercise on the same day. After performance of acute strenuous exercise on the next day, third sample was obtained. A sample was obtained after one month of scheduled regular moderate exercise on the last day after exercise. Baseline sample just before acute strenuous exercise and just before performance of exercise on the last day of one month regular moderate exercise was not obtained. The other part of the samples collected from each individual was aliquoted and stored at - 400C till further analysis.
Plasma sample was used to estimate the levels of cytokine IFN-γ, by using ELISA (Enzyme linked Immuno Sorbent Assay) method. ELISA was performed using DuoSet ELISA development system as per the manufacturer’s instructions (R&D systems, USA). Briefly, polystyrene microtiter plates (NUNC, U16 Maxisorp type, Denmark) were coated with monoclonal capture antibody (antihuman IFN-γ) obtained from mouse (R&D systems, USA) and incubated at 4°C overnight. The following day, the plates were blocked and then incubated for 2 hours with plasma. This was followed by addition of corresponding biotinylated detection antibody obtained from goat (R&D systems, USA) and incubated for two hours. Strepatavidin, horseradish peroxidise conjugate and then, 3,3’,5,5’- tetramethylbenzidine substrate (Bangalore Genie, India) followed this incubation. The reaction was stopped using 2 N sulphuric acid and optical density (O.D) reading was taken at 450nm (Organon Teknika Microwell system, Reader 230s, Germany). All the experiments were conducted in duplicates. A standard curve was obtained based on the standards provided by the manufacturer. The results were expressed as concentration of cytokines (in pg/ml) read from the standard curve (concentration in range: minimum of 5 pg/ml, to maximum of 150 pg/ml).
Statistical Analysis
The statistical analysis was carried out using SPSS software version 18.0 (SPSS Inc. Chicago, USA). The variables in the data were summarized as Mean ± SD. Friedman test has been used for analysing the difference in IFN-γ values in-between the different grades of exercise in the group. Since the SD was large, analysis was done after logarithmic transformation of the values obtained.
Results
Eighteen healthy volunteers in the age group 18 to 25 (mean: 20.94) were taken for the study. IFN-γ levels were studied with different grades of exercises. There was a significant fall in the levels of this cytokine with both acute moderate exercise (p=0.004) and acute strenuous exercise (p=0.001) compared to baseline value. There was a significant drop in its levels after acute strenuous exercise when compared to moderate exercise (p=0.033). The rise of IFN-γ after one month of regular moderate exercise was also significant compared to baseline value (p=0.001). That is, the IFN-γ level shot up after the bout of moderate exercise on the last day of one month of regular moderate exercise regime [Table/Fig-1,2and3].
Effect of graded exercise on IFN-γ levels (pg/ml).
| Mean ± SD | Median | Minimum | Maximum | Log transformation Mean± SD |
|---|
| Age (Yrs) | 20.94±1.76 | 20.50 | 18.00 | 25.00 | --- |
| BMI (kg/m2) | 21.65±1.84 | 21.23 | 19.00 | 24.64 | --- |
| Baseline IFN-γ | 54.56±28.54 | 44.00 | 17.00 | 105.00 | 1.68±0.23 |
| Moderate exercise IFN-γ* | 28.94±38.46 | 18.00 | 5.00 | 131.00 | 1.34±0.24* |
| Strenous exercise IFN-γ* | 20.06±16.96 | 15.50 | 6.00 | 75.00 | 1.18±0.33* |
| One month regular moderate exercise IFN-γ* | 106.33±21.51 | 120.00 | 66.00 | 125.00 | 2.02±0.09* |
*p < 0.05: IFN-γ is statistically significant between different grades of exercise
Changes in the IFN-γ levels (pg/ml) with different grades of exercise
Changes in the IFN-γ levels (pg/ml) with different grades of exercise
Discussion
Sudden bouts of physical activity are deleterious to health [18]. The immune system, in many ways, reacts similarly to the sudden bouts of physical activity and injury [19]. Pro-inflammatory cytokines like interleukin-6 are released is response to acute and strenuous exercise [20,21]. Prolonged strenuous exercise decreases the percentage of T cells in circulation [22]. Increased levels of pro-inflammatory cytokines like interleukin-6 and tumour necrosis factor alpha are deleterious to health [23].
IFN-γ has anti-inflammatory properties. What we infer from this study is that IFN-γ levels increased after a bout of moderate exercise and there was a significant decrease following a bout of strenuous exercise and increased significantly compared to baseline levels when compared with one month of scheduled regular moderate exercise done on a daily basis; that is, IFN-γ levels did shoot up after the single bout of moderate exercise on the last day of one month of scheduled moderate exercise when compared to single bout of moderate exercise without accustumisation to regular moderate exercise, in the same individuals. So it can be postulated that in individuals who adhere to regular moderate exercise, the sudden and drastic fall in the anti-inflammatory cytokine may not occur if such individuals were to get involved in bouts of unaccustomed physical activity. It may induce a ‘buffering capacity’ or an ‘adaptive cytokine response’. This may be beneficial during sudden bouts of physical activity in normal course of life to tolerate those physical stresses better.
Regular moderate exercise is beneficial for maintaining good health and improving the immune status [3–7]. Since this study shows a rise in IFN-γ levels with regular moderate exercise, a rise in the plasma levels of IFN-γ should also be beneficial for health and immunity. IFN-γ is an anti inflammatory cytokine, so its decreased production leads to unnecessary inflammation and tissue damage [24]. Thus regular moderate exercise seems to modulate its release and increases its levels to that necessary for human body to maintain good health.
Mental stress is also known to decrease plasma level of IFN-γ [25]. The adaptive cytokine response may also help individuals adhering to regular moderate exercise to cope with bouts of psychological stresses encountered in daily life: In these individuals, the levels of IFN-γ may not fall as much as it would decrease in those not performing regular exercise, facing the same level of mental stress [26].
The mechanism behind this is not clearly understood and needs further clarification.
Several studies have shown that patients of various diseases and disorders like atherosclerosis, coronary artery disease and diabetes mellitus have elevated levels of interleukin-6 (IL-6) and lower levels of IFN-γ [24]. Stress by way of bursts of physical activity in day-to-day life, in such patients increases their levels much further and leads to exacerbation of the disease. It can be postulated that the drastic rise in IL-6 fall in IFN-γ with bursts of physical activity or with ‘acute on chronic infections’ tends to flatten if such patients follow a regular moderate exercise regimen.
Certain autoimmune disorders like rheumatoid arthritis are associated with increased plasma levels of pro inflammatory cytokines like IL-6, which increase joint inflammation [27]. The anti-inflammatory properties of IFN-γ have been demonstrated both in vitro and in vivo. IFN-γ inhibits integrin mediated adhesion and migration of T-cells in vitro. Flashion et al., have demonstrated that when IFN-γ was administered to naïve T-cells and injected to mice, such T-cells did not home in onto the lymph nodes compared to the T-cells not treated with IFN-γ [1]. IFN-γ decreases eosinophil infiltration in a dose dependent manner [28]. Till date very few studies have been undertaken to study the effects of physical stress/exercise on levels of IFN-γ in human subjects. Regular moderate exercise may benefit patients suffering from inflammatory diseases and auto-immune disorders by bringing down the levels of pro-inflammatory cytokines and increasing the levels of IFN-γ. So we propose that this study has potential for clinical application in future.
*p < 0.05: IFN-γ is statistically significant between different grades of exercise
[1]. Flaishon L, Topilski I, Shoseyov D, Hershkoviz R, Fireman E, Levo Y, Cutting edge: anti-inflammatory properties of low levels of IFN-gammaJ Immunol 2002 168(8):3707-11. [Google Scholar]
[2]. Janice K, Kiecolt-Glaser Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Chronic stress and age-related increases in the proinflammatory cytokine IL-6Proc Natl Acad Sci. U S A 2003 100(15):9090-5. [Google Scholar]
[3]. Blair SN, Kohl HW, Gordon NF, Paffenbarger RS, How much physical activity is good for health?Annu Rev Public Health 1992 13:99-126. [Google Scholar]
[4]. Mackinnon LT, Chronic exercise training effects on immune functionMed Sci Sports Exerc 2000 32(7):369-76. [Google Scholar]
[5]. Pool AJ, Axford JS, The effects of exercise on the hormonal and immune systems in rheumatoid arthritisRheumatology 2001 40:610-4. [Google Scholar]
[6]. Akimoto T, Kumai Y, Akama T, Hayashi E, Murakami H, Soma R, Effects of 12 months of exercise training on salivary secretory IgA levels in elderly subjectsBr J Sports Med 2003 37(1):76-9. [Google Scholar]
[7]. Kentrou P, Ciestak T, MacNeil M, Vintinner A, Plyley M, Effect of moderate exercise on salivary immunoglobin A and infection risk in humansEur J Appl Physiol 2002 87(2):153-8. [Google Scholar]
[8]. Peake JM, Suzuki K, Wilson G, Hordern M, Nosaka K, Mackinnon L, Exercise-induced muscle damage, plasma cytokines, and markers of neutrophil activationMed Sci Sports Exerc 2005 37(5):737-45. [Google Scholar]
[9]. Merino DG, Chennaoui M, Burnat P, Drugon C, Guezennec CY, Immune and hormonal changes following intense military trainingMil Med 2003 168(12):1034-8. [Google Scholar]
[10]. Jankord R, Jemiolo B, Influence of physical activity on serum IL-6 and IL-10 levels in healthy older menMed Sci Sports Exerc 2004 36(6):960-4. [Google Scholar]
[11]. Haahr PM, Pedersen BK, Fomsgaard A, Tvede N, Diamant M, Klarlund K, Effect of physical exercise on in vitro production of interleukin 6, tumour necrosis factor alpha, interleukin 2 and interferon-gammaInt J Sports Med 1991 12(2):223-7. [Google Scholar]
[12]. Kimura H, Suzui M, Nagao F, Matsumato K, Highly sensitive determination of plasma cytokines by time resolved fluoroimmunoassay; effect of byccycle exercise on plasma level of interleukin – 1 – alpha, tumour necrosis factor alpha and interferon gammaAnal Sci 2001 17(5):593-7. [Google Scholar]
[13]. Pal GK, Pal P, Text Book of Practical Physiology 2001 1stsub editionChennai (India)Orient Longman Limited [Google Scholar]
[14]. Singh SJ, Morgan MDL, Scott S, Walters D, Hardman AE, Development of a shuttle walking test of disability in patients with chronic airways obstructionThorax 1992 47:1010-24. [Google Scholar]
[15]. Leger LA, Lambert J, A maximal multistage 20m shuttle run test to predict VO2maxEur J Appl Physiol 1982 49:1-12. [Google Scholar]
[16]. Dyer CAE, Singh SJ, Stockley RA, Sinclair AJ, Hill SL, The incremental shuttle walking test in elderly people with chronic airflow limitationThorax 2002 57:34-8. [Google Scholar]
[17]. Pratt RK, Fairbank JCT, Virr A, The reliability of the Shuttle Walking Test, the Swiss Spinal Stenosis Questionnaire, the Oxford Spinal Stenosis Score, and the Oswestry Disability Index in the assessment of patients with lumbar spinal stenosisSpine 2002 27(1):84-91. [Google Scholar]
[18]. Castell LM, Poortmans JR, Leclercq R, Brasseur M, Duchateau J, Newsholme EA, Some aspects of the acute phase response after a marathon race, and the effects of glutamine supplementationEur J of Appl Physiol 1996 75(1):47-53. [Google Scholar]
[19]. Northoff H, Berg A, Weinstock C, Similarities and differences of the immune response to exercise and trauma: the IFN-gamma conceptCan J Physiol Pharmacol 1998 76(5):497-504. [Google Scholar]
[20]. Pedersen BK, Steenberg A, Exercise and hypoxia: effects on leukocytes and interleukin-6 - shared mechanisms?Med Sci Sports Exerc 2002 34(12):2004-13. [Google Scholar]
[21]. Northoff H, Weinstock C, Berg A, The cytokine response to strenuous exerciseInt J Sports Med 1994 15(3):167-71. [Google Scholar]
[22]. Steensberg A, Toft AD, Bruunsges H, Halkjaer KJ, Pedersen BK, Strenuous exercise decreases the percentage of type 1 T cells in the circulationJ Appl Physiol 2001 91(4):1708-12. [Google Scholar]
[23]. Fauci AS, Braunwald E, Isselbacker KJ, Wilson JD, Martin JB, Kasper DL, Harrison’s Principles of Internal Medicine 1998 14th editionNew York (US)McGraw- Hill [Google Scholar]
[24]. Meager T, The Molecular Biology of Cytokines 1998 1st editionChichester (UK)John Wiley & Sons [Google Scholar]
[25]. Glaser JKK, Preacher KJ, Robert C, Atkinson MC, Malarkey WB, Glaser R, Chronic stress and age-related increases in the proinflammatory cytokine IL-6Proc Natl Acad Sci USA 2003 100(15):9090-5. [Google Scholar]
[26]. Lloyd A, Gandevia S, Brockman A, Hales J, Wakefield D, Cytokine production and fatigue in patients with chronic fatigue syndrome and healthy control subjects in response to exerciseClin Infect Dis 1994 18(1):142-6. [Google Scholar]
[27]. Cotran RS, Kumar V, Robbins SL, Robbins pathologic basis of disease 1994 5th editionMassachusetts (Boston)WB Saunders Company [Google Scholar]
[28]. Iwamoto I, Nakajima H, Endo H, Yoshida S, Interferon γ regulates antigen-induced eosinophil recruitment into the mouse airways by inhibiting the infiltration of CD4+ T cellsJ Exp Med 1993 177:573 [Google Scholar]