Morphological Study of Dendritic Cells in Human Cervix by Zinc Iodide Osmium Method
Suganthy Rabi1, J Lionel2, Inbam Indrasingh3
1Professor, Department of Anatomy,Christian Medical College,Vellore, India.
2Professor, Department of Obstetrics & Gynaecology,Christian Medical College,Vellore, India.
3Professor, Department of Anatomy,Christian Medical College,Vellore, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Suganthy Rabi, Department of Anatomy, Christian Medical College, Vellore-632002, India.
Phone: 919442978775,
E-mail: suganthyrabi@cmcvellore.ac.in
Background: Dendritic cells (DCs) are a heterogeneous population of antigen presenting cells that have been identified in several tissues including the female reproductive organs. The aim of the present study is to demonstrate the morphological differences of dendritic cells in normal human exocervix using the Zinc Iodide Osmium (ZIO) procedure.
Materials and Methods: Normal cervical tissues obtained from nine patients who underwent abdominal hysterectomies for various ailments were processed for histochemical study. Six microns thick serial sections were taken and viewed under a light microscope. The diameters of the cells were measured under a magnification of 40x using the Cellsens image analysing software and analysed using SPSS version 16.
Results and Conclusion: In the normal human exocervix, a greater density of ZIO-positive DCs was noted in the epithelium and subepithelium and their distribution was not uniform. In some areas of epithelium, the ZIO-positive cells in the basal layer showed a typical dendritic morphology, while the cells in the intermediate and superficial layers were nondendritic polygonal cells. Intraepithelial capillaries were noted, which were surrounded by ZIO-positive nondendritic polygonal cells. There was significant difference in the mean diameters of typical DCs (8.61±1.86 μm) and nondendritic polygonal cells (10.97±1.93 μm). In the subepithelium the DCs had typical morphology and their distribution varied. ZIO positive DCs were noted in the epithelium and cervical glands of endocervix also. In conclusion, the human cervix has different subsets of ZIO positive DCs with varied distribution. Their functional role has yet to be defined.
Dendritic cells, Human exocervix, Langerhans cells, Morphology, ZIO
Introduction
Dendritic cells are potent immunostimulatory cells [1]. They are often present in tissues with high antigenic exposure, such as skin and mucosa, where they capture and process antigens. They include Langerhans cells, interdigitating cells, veil cells and follicular dendritic cells. The properties of DCs include the ability to capture, process and present foreign antigens, to migrate to lymphoidrich tissue, and to stimulate innate and adaptive antigen-specific immune responses [2] .
Several markers have been used to identify DCs. Depending on the microenvironment, DCs express several surface markers and secrete cytokines such as IL-1, IL-2 and TNF alpha [3] . It is known that epidermal Langerhans cells react with osmium-zinc iodide (ZIO) mixtures; and hence they can be visualized with this histochemical method. Previously ZIO method has been successfully used for the demonstration of Langerhans cells with both light and electron microscopy [4,5] .
The female reproductive tract is considered to be a part of the mucosal immune system. The local immune system in the female reproductive tract encounters commensal bacteria on the epithelial surface and pathogenic microorganisms that multiply in the mucosa [6] . The lower reproductive tract including the vagina and exocervix is lined by stratified squamous epithelium. This epithelium offers a physical barrier for infection. The mucus secreting cells present in the mucosa prevent entry of harmful bacteria [7] . In addition to this, defense at mucosal surface is mediated through humoral and cell mediated immunity. DCs play a major role in the host defense mechanism. Their presence in human reproductive organs has been demonstrated earlier. Although their role in infections like HIV [8,9] , Chlamydia trachomatis [10] and in association with hormones [11] have been studied earlier, their distribution and morphology has not been extensively studied. The present study demonstrates different morphologies of ZIO-positive DCs in the normal human exocervix.
Materials and Methods
Ethical approval for the study was obtained from the Institutional Review Board, Christian Medical College, Vellore, India. The study was done for a period of two yrs. Normal cervical tissue was obtained from nine patients who underwent total abdominal hysterectomy for fibroid uterus, dysfunctional uterine bleeding and adenomyosis uterus at the Christian Medical College Hospital, Vellore, India after getting informed consent. The tissue pieces were immersed in a solution of veronal-buffered Zinc Iodide-Osmium tetroxide at pH 7.4 [5] for 60 hours at 4oC in the dark, washed in distilled water, dehydrated in a graded ethanol series, cleared in xylene, and embedded in paraffin wax. Serial sections of six microns thick were cut and transferred to glass slides, deparaffinised, mounted in DPX without counterstaining and viewed under the light microscope, Olympus BX43. The diameters of the cells were measured under a magnification of 40x using the Cellsens image analysing software and analysed using SPSS version 16.
Results
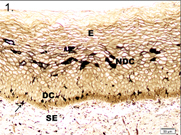
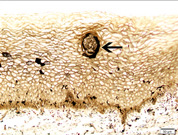
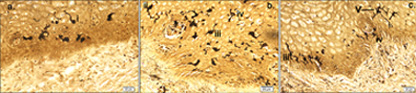
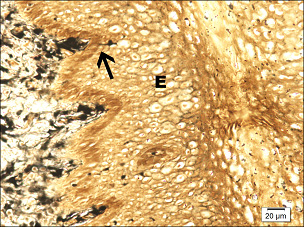
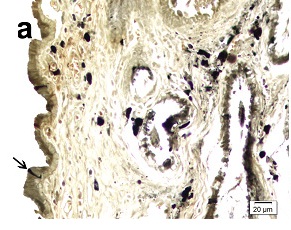
Paraffin embedded 6 μm sections of normal exocervix showed darkly stained, ZIO positive cells in the epithelium and in subepithelium. There was regional variation in their distribution and morphology. ZIO-positive cells with two different morphologies were present. Cells situated in the basal and suprabasal layers were small and had a typical dendritic morphology. The intermediate layer of the epithelium showed cells, which were large and polygonal in shape [Table/Fig-1] . In some areas, there were large polygonal cells in the superficial layer. The diameter of typical DCs ranged from 3.64 to 13.34 μm with a mean diameter of 8.61 ± 1.86 μm. The diameter of nondendritic cells ranged from 7.50 to 17.52 μm with a mean diameter of 10.97 ±1.93 μm. There was significant difference in the mean diameters of typical DCs and nondendritic cells (p<0.0001) [Table/Fig-2]. Intraepithelial capillaries were noted and they were surrounded by ZIO-positive cells. The polygonal ZIO positive cells, as they approached the capillaries, flattened out and completely surrounded the blood vessels [Table/Fig-3]. In some areas, there were typical DCs in the basal and suprabasal region but no polygonal cells in the intermediate layer. In the region of the mucosal fold, ZIO-positive cells differed in distribution and in morphology. In certain regions of the mucosal fold, there were typical dendritic cells in all layers. In certain other areas, there were small ZIO-positive cells in the basal and suprabasal layers with no definitive dendritic pattern. In the epithelium of exocervix, all five types of dendritic cells as described by Figureroa & Caorsi could be identified[Table/Fig-4a,b,c]. In certain regions of the mucosal fold, a high density of ZIO-positive DCs was present in the subepithelium while certain areas were devoid of DCs. There was evidence that the DCs had migrated from the subepithelium to epithelium [Table/Fig-5].
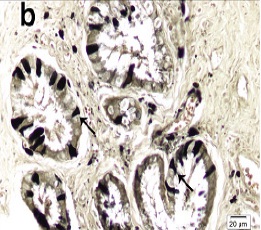
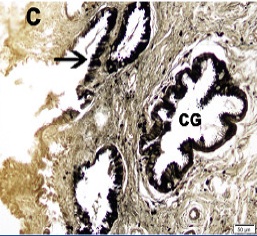
In the endocervix, there were triangular ZIO positive cells along the columnar epithelium and in the glands of cervix. The cells in the uterine glands were columnar or triangular in shape with their single process directed towards the lumen [Table/Fig-6a,6b] . In the case of endocervicitis, there were numerous ZIO positive cells in the cervical glands [Table/Fig-6c] .
The nonkeratinized stratified squamous epithelium (E) of cervix stained with ZIO. Cells with typical dendritic morphology (DC) are seen in the basal and suprabasal region. The midepithelium has nondendritic polygonal ZIO positive cells (NDC). Arrow indicates a dendritic cell in the subepithelium (SE)
Comparison of size of typical dendritic cell and nondendritic ZIO positive DCs in cervical epithelium in chronic cervicitis using Student’s t-test (n=100)
| Tissue | Minimum in μm | Maximum in μm | Mean in μm | Standard Deviation | p-value |
| Typical DC | 3.64 | 13.34 | 8.61 | 1.87 | <.0001 |
| Nondendritic cell | 7.50 | 17.52 | 10.97 | 1.94 |
Arrow indicates ZIO positive an intraepithelial capillary surrounded by nondendritic ZIO positive cells (NDC) in the cervical epithelium
Various types of DCs seen in the cervical epithelium. i – Type I cell with a single unbranched process; ii- Type II cell with single branched dendrite; iii- Type II cell having 2 dendritic processes; iv – Type IV cell having 3 or more processes; v – Type V cells having 3 or more dendrites with further branching
Arrow indicates a DC migrating from the subepithelium into the epithelium (E)
Arrow indicates a triangular ZIO positive cell in the columnar epithelium of the endocervix.
Arrows indicate triangular ZIO positive cells in the glandular epithelium of cervix.
Endocervix of a patient with endocervicitis. Arrow indicates the ZIO positive cells in the epithelium. Note the abundant number of ZIO positive cells in the cervical glands (CG)
Discussion
DCs, derived from bone marrow are the most potent antigen presenting cells and are capable of activating naïve T lymphocytes and thus initiating adaptive immune responses [12]. They play a vital role in the host defense mechanism of the lower reproductive tract. Several authors have studied the morphology and number of DCs in normal, Human papilloma virus (HPV)-infected and cancerous cervix. The presence of Langerhans cells in the normal human exocervix was first demonstrated in 1968 by ultrastructural studies [13,14]. Langerhans cells appear to form a relatively constant component of both the transformation zone and exocervical epithelium [15]. Their presence in the intermediate and basal layers of the exocervical epithelium has been previously reported [16]. It was reported earlier that there was an increased density of LCs towards the basement membrane and the dendritic aspect was more prominent in the superficial layers using immunohistochemistry [17]. In the present study, the ZIO positive DCs with typical morphology were predominantly seen in the basal and suprabasal region and their dendritic nature was more prominent in the basal layers unlike that has been reported earlier. Figureroa & Caorsi [5] studied Langerhans cells using the zinc iodide-osmium technique. They described five types of Langerhans cells on the basis of the number of cell processes and the degree of branching of these processes under light microscopy. In the present study, all the five types of Langerhans cells have been demonstrated.
While the DCs with typical dendritic morphology was observed in the epithelium, the intermediate and the superficial layers had many ZIO positive polygonal shaped cells with no dendritic processes. They were seen migrating towards the intraepithelial capillaries and surrounding them. Although these cells have not been described earlier in the cervix, they have been described previously in the intermediate layer of the stratified squamous epithelium of the human tonsil [18] and in the lip of the Bonnet monkey [19]. These polygonal cells were not evenly distributed in all regions. It is not known whether they belong to the monocyte-macrophage-histiocyte family. The function of these cells remains unknown. These cells could be viewed as nondendritic accessory antigen-presenting cells. In view of their migration towards intraepithelial capillaries, they could be considered as maturing DCs that modify their morphology and present antigens to the lymphocytes in the blood vessels. DC maturation is associated with decreased antigen uptake and increased ability to activate T-cells [20].
The distribution of ZIO-positive DCs in the subepithelium of exocervix was not uniform. In the mucosal fold region, some areas had abundant ZIO positive DCs while other regions were devoid of 3these cells. In this study, there is evidence that DCs migrate from the subepithelium to epithelium where they come in contact with the antigens.
ZIO positive DCs in endocervix was described for the first time in the present study. They resemble the ZIO positive cells demonstrated in the crypts of human colon [21], human ileum [22], human appendix [23] and uterine tube [24]. Though there is a challenge to figure out the functional role of these cells, the increased density of these ZIO positive cells in endocervitis indicate that these cells were actively engaged in mucosal immune system of the reproductive tract.
Conclusion
It has been shown in the present study that the normal human cervix has ZIO positive cells with different morphologies and their distribution is not uniform. For the first time, nondendritic ZIO positive DCs were demonstrated in the epithelium of cervix. Although, ZIO has been used as the specific marker for Langerhans cells by various authors in the past, the morphology of ZIO positive DCs in human cervix indicate that all ZIO positive cells may not be Langerhans cells and the ZIO positive cells represent a heterogeneous antigen presenting cell population with different functions. The functions of nondendritic ZIO positive cells are yet to be determined and further studies are required to understand the functional role to these cells. The demonstration of different subtypes of ZIO positive DCs in the present study could have important implications and would help to further the knowledge and understanding of DCs.
Acknowledgement
The authors gratefully acknowledge the Indian Council of Medical Research for funding this project.
[1]. RM Steinman, The dendritic cell system and its role in immunogenicityAnnual Rev Immunol 1991 9:271-96. [Google Scholar]
[2]. R Foley, R Tozer, Y Wan, Genitically modified dendritic cells in cancer therapy: Implications for transfusion medicineTransfus Med Rev 2001 15:292-304. [Google Scholar]
[3]. S Coronata, GE Laguens, OM Spnelli, MA Salas, W Di Girolamo, Dendritic cells and their role in pathology.Medicina (B Aires). 1998 58:209-18. [Google Scholar]
[4]. G Niebauer, WS Krawczyk, RL Kidd, GF Wilgram, Osmium Zinc – Iodide reactive sites in the epidermal Langerhans cells.J cell Biol 1969 43:80-9. [Google Scholar]
[5]. CD Figueroa, I Caorsi, Ultrastructural morphometric study of the Langerhans cells in the normal human exocervixJ Anat 1980 131:669-82. [Google Scholar]
[6]. EL Johansson, A Rudin, L Wassén, J Holmgren, Distribution of lymphocytes and adhesion molecules in human cervix and vaginaImmunology. 1999 96(2):272-7. [Google Scholar]
[7]. N Iijima, JM Thompson, A Iwasaki, Dendritic cells and macrophages in the genitourinary tract.Mucosal Immunol 2008 1(6):451-9. [Google Scholar]
[8]. G Levi, J Feldman, S Holman, A Salarieh, HD Strickler, S Alter, Relationship between HIV viral load and Langerhans cells of the cervical epitheliumJ Obstet Gynaecol Res 2005 31:178-84. [Google Scholar]
[9]. BL Shacklett, Cell-mediated immunity to HIV in the female reproductive tract. J. ReprodImmunol. 2009 83:190-5. [Google Scholar]
[10]. T Agrawal, V Vats, PK Wallace, A Singh, S Salhan, A Mittal, Recruitment of myeloid and plasmacytoid dendritic cells in cervical mucosa during Chlamydia trachomatis infection.Clin. Microbiol Infect 2009 15:50-9. [Google Scholar]
[11]. CR Wira, RM Rossoll, C Kaushic, Antigen-presenting cells in the female reproductive tract: influence of estradiol on antigen presentation by vaginal cells.Endocrinology. 2000 141:2877-85. [Google Scholar]
[12]. P Guermonprez, J Valladeau, L Zitvogel, C Thery, S Amigorena, Antigen presentation and T cell stimulation by dendritic cells.Annu Rev Immunol 2002 20:621-67. [Google Scholar]
[13]. MS Younes, EM Robertson, SA Bencosme, Electron microscope observations on Langerhans cells in the cervix.American J of Obstetrics and Gynecology 1968 102:397-403. [Google Scholar]
[14]. M Hackemann, C Grubb, KR Hill, The ultrastructure of normal squamous epithelium of human cervix uteriJ ultrastruct Res 1968 22:443-57. [Google Scholar]
[15]. WA Poppe, M Drijkoningen, PS Ide, JM Lauwervns, FA Van Assche, Lymphocytes and dendritic cells in the normal uterine cervix. An immunohistochemical studyEur J Obstet Gynecol Reprod Biol 1998 81(2):277-82. [Google Scholar]
[16]. NS Uchimura, JC Ribalta, J Focchi, MJ Simoes, TT Uchimura, ES Silva, Evaluation of Langherhans` cells in human papilloma virus- associated squamous intraepithelial lesions of the uterine cervix.Clin Exp Obest Gyneco 2004 31:260-2. [Google Scholar]
[17]. AE Morelli, G Di Paola, L Fainboim, Density and distribution of Langerhans cells in the human uterine cervix.Arch Gynecol Obstet 1992 252:65-71. [Google Scholar]
[18]. G Chandi, I Indrasingh, G Sridharan, Dendritic cells in the tonsillar epitheliumJ Anat Soc India. 1998 37:181-5. [Google Scholar]
[19]. Indrasingh S Abraham, SK Vettivel, Zinc Iodide Osmium positive cells and dendritic cells in stratified squamous epithelium of lip, tongue and oesophagus of Bonnet Monkey (Macaca Radiata)J Anat Soc India. 2001 50:34-6. [Google Scholar]
[20]. G Stingl, PR Bergstresser, Dendritic cells: a major story unfoldsImmunol Today 1995 16(7):330-3. [Google Scholar]
[21]. I Indrasingh, S Koshy, S Vettivel, Morphology and distribution of Human colonic dendritic cells: A light microscopic Zinc Iodide-Osmium studyJ. Anat Soc India. 2003 52:7-11. [Google Scholar]
[22]. S Koshy, I Indrasingh, S Vettivel, Dendritic cells in the human ileum: A light microscopic Zinc Iodide-Osmium study.Eur J Anatomy 2003 7:127-30. [Google Scholar]
[23]. J Suganthy, I Indrasingh, J Lionel, Demonstration of ZIO positive Langerhans cells in the normal and the postpartum human fallopian tubesJ Anat Soc India 2006 55(2):1-4. [Google Scholar]
[24]. S Rabi, I Indrasingh, S Koshy, Distribution of zinc iodide-osmium positive dendritic cells in the human appendix.Eur J Anat 2006 10:15-20. [Google Scholar]