Background and Objective: AmpC β lactamases are one of the important causes of drug resistance in gram negative bacteria. Failure to detect these enzymes in the laboratory has contributed to therapeutic failures but there are till date no standard guideline available. This study was therefore undertaken to evaluate three phenotypic laboratory tests and the inhibitors used in two of the tests to detect AmpC β lactamases produced by E. coli and Klebsiella species as they are most commonly isolated organisms.
Methods: E. coli and Klebsiella isolates from different clinical samples were tested for ESBLs production as per CLSI guidelines and excluded from the study. The non-ESBLs isolates were then screened for AmpC β lactamases production, by cefoxitin and then confirmed by three different methods, i.e., Disc Potentiation Test (DPT) , Double Disc Synergy Test (DDST) and Modified Three Dimensional Test (M3DT) which in the absence of molecular methods, was taken as the gold standard. Boronic acid and cloxacillin were used as inhibitory agents in the Disc Potentiation and Double Disc synergy Tests.
Results: A total of 2,933 isolates were tested out of which 165 isolates were detected as non ESBLs producers,135 (81.82%) when screened for AmpC β lactamases based on resistance to cefoxitin were labelled as positive. 30 (18.18%) cefoxitin sensitive isolates were labelled as probably non AmpC producers . M3DT, in addition to detecting all the 135 (100%) cefoxitin resistant isolates, also detected 5 (16.67%) cefoxitin sensitive isolates as AmpC producers. Other phenotypic tests, DPT and DDST with different inhibitors like boronic acid and cloxacillin in different potencies were all found to be less sensitive. The best results among these two methods were obtained with DDST using cloxacillin 500μg.
Conclusion: In the absence of recommended guidelines for AmpC detection, the study reports, among the tests performed, M3DT as the best phenotypic method for AmpC confirmation, as it is not only the most sensitive but also specific test for AmpC as it rules out the resistance due to other mechanisms like the porin channel.
Introduction
The development of drug resistance against the β lactam drugs by the gram negative organisms has been the greatest set back in the treatment of patients suffering from infection with such isolates and amongst these, the production of AmpC β-lactamases has emerged as one very important mechanism and yet, it has not been studied as extensively as other β lactamases.
AmpC β lactamases are enzymes which belong to class C of Ambler [1] and group I of Bush et al., [2]. These enzymes confer resistance to a wide variety of β lactam drugs including β lactamase inhibitors like clavulanic acid , sulbactum and tazobactum to which class A enzyme (ESBLs) are sensitive [3]. They are sensitive to cefepime , cefpirome and carbapenems.
Both Extended Spectrum β lactamases (ESBLs) and Amp C β lactamases (AmpC) may be produced together by an organism but generally the effect of plasmid mediated AmpC β lactamases masks the effect of ESBLs which may then be wrongly reported as ESBL negative Failure to detect these enzymes has contributed to their uncontrolled spread and many times to therapeutic failures. While Clinical and Laboratory Standard Institute (CLSI) recommendations are available for detection of ESBLs producing isolates of E. coli and Klebsiella species, there are no such guidelines for detection of AmpC β lactamases [4]. Detecting AmpC β lactamases at molecular level is technically difficult and the phenotypic tests for AmpC detection are not well defined , though there are individual reports on confirmatory test like the Disc Potentiation Test (DPT) [5,6], Double Disc Synergy Test (DDST) [5,6], AmpC Disc Test [7] and Modified Three Dimentional Test (M3DT) [8]. However, there is no consensus either on the type of phenotypic test or on the suitable AmpC inhibitors that should be used with cephamycins to detect these enzymes [9]. The general principle for detection of these enzymes is first to perform the screening test with cefoxitin and then use the confirmatory tests as mentioned above. However, consensus is still to built up on the best test for the purpose including the inhibitors to be used. The present study was therefore undertaken with the objectives (i) to compare the above three confirmatory methods with respect to detection of AmpC β lactamases in E. coli and Klebsiella species which are the most commonly isolated organisms and (ii) to compare simultaneously the results of two inhibitors, i.e., boronic acid (400μg) and cloxacillin (300 ug and 500 ug) used in DPT and DDST.
Materials and Methods
The study was conducted in the Department of Microbiology, MGIMS, Sevagram from 2007-2009 and was duly approved by the Ethics Committee. Clinical isolates of E. coli and Klebseilla species recovered from clinical samples such as urine, pus, wound swab, vaginal swab, blood, fluids, CSF, tissue, sputum, drain, tracheal swab during routine testing were processed. All isolates were identified first by standard method and then based on their resistance to 3rd generation cephalosporin like, ceftazidime (30μg) and cefotaxime (30μg), they were processed further for the detection of the ESBL by Disc Potentiation Test [4]. The organisms that showed potentiation with clavulanic acid were classified as ESBLs producers and others were separated out as non-ESBLs producers. These were then further subjected to special tests for detection of AmpC β lactamases. Screening was done by using cefoxitin (30μg) disc. Any isolates showing zone of inhibition ≤ 18mm was considered as resistant to cefoxitin and was labelled as probable Amp-C producer. However, whether resistant or sensitive all were subjected to confirmation with. Disc Potentiation Test (DPT)[5,6], Double Disc Synergy Test (DDST) [5,6] and Modified Three Dimensional Test (M3DT) [8].
1. Detection of AmpC β Lactamases by Disc Potentiation Test (DPT): [5,6]:
The Disc Potentiation Test was performed on all non ESBLs isolates using three different enzymes inhibitors, i.e., boronic acid 400μg (10μl)[5] cloxacillin 300μg (10μl) and 500μg (10μl) [6]. The test is based on the principle that each of these substances by inactivating the AmpC enzyme produced by the organism, potentiate the inhibitory effect of the antibiotic on the organism. The test was performed separately for each inhibitor. A lawn culture of the test organisms was made. Two discs each of ceftazidime (30μg) and cefotaxime (30μg) were placed on the lawn culture distant (25 mm) from each other. A 10μl of freshly prepared enzyme inhibitor under test was added to one of the two disc of each cephalosporin and the zone of inhibition was measured. An increase in zone by ≥ 5mm around the disc with inhibitor compared to the zone formed by the disc without the inhibitor, confirmed the test organism to be an AmpC producer as the inhibitor potentiated the inhibitory effect of the cephalosporins by inactivating the AmpC enzyme. The readings taken separately for all the three inhibitors for each test organism were noted and compared.
2. Detection of AmpC β lactamase by Double Disc Synergy Test (DDST ) [5,6]:
Double Disc Synergy Tests using boronic acid 400 μg (10μl) [5] , cloxacillin 300(10μl) μg & 500 μg (10μl) [6]were also put up for all non-ESBLs producing strains using each of the above mentioned inhibitors of AmpC β lactamases individually. Using Mueller Hinton plate for each test organism, a lawn culture was made, the disc of the inhibitor was placed in the centre and the distance between this disc and cefotaxime (30μg) and ceftazidime (30μg), one on either side from centre to centre, was kept as 15 mm. After overnight incubation at 370C, expansion of inhibitory zone of either one or both, ceftazidime and cefotaxime, towards the inhibitory discs was interpretated as positive results for production of AmpC β lactamases by the isolates [Table/Fig-1].
3. Modified Three Dimensional Tests (M3DT) [8]
Non-AmpC producing ATCC E. coli 25,922 was used as indicator strain in this test. Lawn culture from the growth of this organism with turbidity of 0.5 McFarland was prepared on Mueller Hinton agar plate and cefoxitin 30 μg disc was placed in the centre of the plate. A linear slit of 3cm length, 3 mm away from the cefoxitin disc, was cut using sterile surgical blade. At the other end of the slit a small circular well was made with the help of debeveled needle No-18 which was found to be more convenient than the recommended Pasteur pipette. The extract of the enzyme AmpC β lactamases was prepared by freezing and thawing the test organism 7-8 times and then subjecting it to centrifugation at 2000 rpm for 15 min. This process released the AmpC β lactamase enzymes into the suspending fluid. A total of 20μl to 30μl of the supernatant containing the extract was then loaded in the prepared well. The plates were kept for 5- 10 minutes to allow the liquid to seep and diffuse into the slit and were then incubated at 370C for 24h. Positive test was read as small heart shaped indentation towards the cephalosporin disc seen at the junction of the slit along the line of inhibition [Table/Fig-2].
In order to confirm the results, the above test was repeated for the same isolate with slight modification. Before loading the well with the extract, a disc of 5μg cloxacillin was added to the tube containing the extract in order to neutralise the AmpC β lactamases enzyme. The test was replaced with the neutralised enzyme extract. Loss of indentation which was seen earlier reconfirmed the AmpC β lactamases production by the test organism.
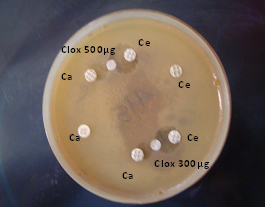
Double Disc Synergy Test (DDST):
• Ce (alone): cefotaxime (30μg) disc showing no zone of inhibition .
• Ca (alone) : ceftazidime (30μg) disc showing no zone of inhibition
• Ce-clox -Ca : cefotaxime (30μg) – cloxacillin (500μg & 300μg) :– ceftazidime (30μg) showing synergistic action of ce and ca towards cloxacillin disc
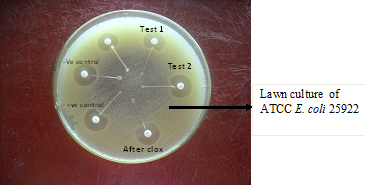
Modified Three Dimensional Test (M3DT) –
• Test-1 and Test -2 : test sample showing heart shaped indentation (Positive test)
• After clox – inhibition of heart shaped indentation after adding cloxacillin 5ug to the enzyme extract.
• Positive control (K.pneumoniae B -2663) - showing heart shaped indentation
• Negative control (ATCC K. pneumonia 700603) – no heart shaped indentation and
• lawn culture of ATCC E. coli 25922.
Comparision of percentage positivity for AmpC β lactamases by the three methods alongwith the assessment of the inhibitors used in DPT and DDST
| Cefoxitin resistant (Cn=R) isolates n=135 | | DPT positive | DDST positive | M3DT Positive |
| Boronic acid (400μg) | Cloxacillin (500μg) | Cloxacillin (300μg) | Boronic acid (400 μg) | Cloxacillin (500μg) | Cloxacillin (300μg) |
| E. coli (n=70) | 21 (30%) | 63 (90%) | 61 (87.14%) | 33 (47.14%) | 67 (95.71%) | 65 (92.86%) | 70 (100%) |
| K.pneumoniae (n=14) | 12 (85.71%) | 13 (92.86%) | 14 (100%) | 8 (57.14%) | 12 (85.71%) | 12 (85.71%) | 14 (100%) |
| K.oxytoca (n=9) | 5 (55.56%) | 8 (88.89%) | 7 (77.78%) | 3 (33.33%) | 5 (55.56%) | 4 (44.44%) | 9 (100%) |
| Other K.species (n=42) | 21 (50%) | 33 (78.57%) | 30 (71.43%) | 19 (45.24%) | 40 (95.24%) | 41 (97.62%) | 42 (100%) |
| Total | 59 (43.70%) | 117 (86.67%) | 112 (82.96%) | 63 (46.67%) | 124 (91.85%) | 122 (90.37%) | 135 (100%) |
| Cefoxitin sensitive isolates (Cn=S), n=30 | Nil | Nil | Nil | Nil | Nil | Nil | 5 (16.67%) |
| Grand total (n=165) | 59 (35.76%) | 117 (70.91%) | 112 (67.89%) | 63 (38.18%) | 124 (75.15%) | 122 (73.94%) | 140 (84.85%) |
| DPT- Disc Potentiation Test ; DDST – Double Disc Synergy test ; M3DT- Modified Three Dimensional Test |
Percentage positivity of AmpC β lactamases among the 2933 isolates tested taking Modified Three Dimensional Test (M3DT) as the gold standards
| Test | E. coli | Different Klebsiella species | Total Klebsiella species | Grand Total |
| | K.pneumoniae | K.oxytoca | Other K. Species | | |
| n=1643 | n=326 | n=196 | n=768 | n =1290 | n=2933 |
| Modified Three Dimensional Test (M3DT) | 73* (4.44%) | 14 (4.29%) | 9 (4.59%) | 44* (5.72%) | 67 (5.19%) | 140 (4.77%) |
| *Including isolates (3E.coli & 2 klebsiella sp) that were detected by M3DT as AmpC producers amongst cefoxitin sensitive isolates |
Results
A total of 2933 isolates of E. coli (1643) and Klebsiella sp (1290) were tested. Out of these, 2453 (83.63%) were observed to be sensitive to 3rd Generation Cephalosporins (3GC), cefotaxime and ceftazidime. All these 480 resistant isolates were further tested for ESBL production by Disc Potentiation Test using cephalosporins and clavulanic acid as inhibitor [4]. Out of these, 315 (65.63%) were found to be ESBL producers while 165 (34.37%) were non ESBL producers and among these 88 (53.33%) were E. coli and 77 (46.67%) were Klebsiella species.
Screening of 165 non ESBL isolates for AmpC β lactamases production with cefoxitin (30ug), revealed 135 (81.82%) isolates i.e. 70 (51.85%) E. coli and 65 (48.15%) Klebsiella species to be resistant to cefoxitin while 30 (18.18%) i.e. 18 (60%) E. coli and 12 (40%) Klebsiella species were sensitive. All the 165 isolates, 135 resistant and 30 sensitive were subjected to different confirmatory tests. [Table/Fig-3] shows the results with cefoxitin resistant strains.It was observed that Modified Three Dimensional Test, was the most sensitive test and detected all the 135 (100%) cefoxitin resistant AmpC β lactamase producing isolates of both E. coli and Klebsiella species. This was followed by DDST with cloxacillin 500μg (91.85%) 7and 300μg (90.37%). DPT with cloxacillin 500μg gave positive results in 86.67% followed by 300μg (82.96%). M3DT showed additional advantage as amongst the 30 cefoxitin sensitive isolates it detected 5 isolates i.e 16.67% (3 E. coli and 2 Klebsiella species) as AmpC producers which were totally missed by DPT and DDST [Table/Fig-3]. In the absence of molecular methods M3DT therefore, was taken as a gold standard for further analysis and comparison with other tests.
Comparison of DPT and DDST with M3DT showed great variation in results not only with respect to E.coli and Klebsiella sp. but also with respect to the inhibitors used for testing the organisms [Table/Fig-3].It was observed that among the inhibitors tested in the two tests, DPT and DDST, boronic acid 400 μg did not give good results with E. coli, Klebsiella oxytoca and other Klebsiella species, though with Klebsiella pneumoniae the percentage positivity with DPT was 85.71%.
The results with cloxacillin were better with DDST than with DPT and both the strengths used in the respective techniques were comparable. However, it was interesting to note that with Klebsiella pneumoniae and Klebsiella oxytoca, both the cloxacillin concentrations showed better results with DPT than with DDST .
Taking M3DT as the gold standard the percentage positivity of AmpC producers in our hospital amongst the total 2933 isolates of E.coli and Klebsiella species tested was found to be 4.77%. The AmpC production in E. coli was 4.44% and for all Klebsiella sp. taken together was 5.19%, with Klebsiella pneumoniae showing 4.29% positivity [Table/Fig-4].
Discussion
Detecting and reporting isolates producing AmpC β lactamases is more difficult than with ESBLs. It was first suggested that ESBLs producing organisms are sensitive to cephamycins like cefoxitin where as, AmpC producers are not and therefore resistance to cefoxitin was suggested as a screening test for AmpC β lactamases [10]. However, soon this test was shown to have limitations. It was observed, though different methods for detection of AmpC β lactamases were used for comparison, that not all cefoxitin resistant isolates were producers of AmpC β lactamases and this range of non producers varied from 20-50% in different studies [8,11]. The resistance in these organisms was considered to be due to the lack of permeation of porins [8]. M3DT has an advantage, that in this test by using the extract of the organism the effect due to porin mechanism is ruled out and the total effect is due to the AmpC β lactamases. In the present study, there was 100% correlation between the cefoxitin resistance and detection of AmpC by M3DT. This was not there with DPT and DDST [Table/Fig-3]; as none of the techniques used in these two tests could elicit AmpC production in all the isolates.
Another feature that was observed in our study was that 16.67% cefoxitin sensitive isolates were also AmpC producers. This phenomenon has also been reported in another study [11] where 71.43 % of the isolates tested were found to be AmpC producers among the cefoxitin sensitive non ESBLs isolates. This could be due to a phenomenon reported earlier [8]where a clinically significant strain of Klebsiella pneumoniae which produced a novel type of AmpC β lactamase with a low level of activity against cefoxitin (MIC 4 ug/ml ) was detected and which, therefore in vitro appeared to be sensitive to cefoxitin but was actually resistant due to the new AmpC production. This isolate was labelled as ACC-1 type belonging to Ambler class C and the gene coding for this was designated as bla ACC-1. Though molecular studies were not done, the 5 isolates, in our study could belong to this group. Detection of such isolates definitely puts the screening test with cefoxitin under the shade.
In the present study, the comparison of confirmatory tests clearly indicated M3DT to be superior to other techniques as it not only detected maximum isolates that were AmpC producers but also detected them among the cefoxitin sensitive isolates which were totally missed by DPT and DDST. Though slightly labour intensive, the test has distinct advantage as it completely does away with the need for any inhibitory agent. Comparison of inhibitory substance like boronic acid and cloxaciliin in the present study has clearly shown extremely variable results not only with both E. coli and Klebsiella species but also amongst the two techniques DPT and DDST. Some studies [5,12] have reported boronic acid 400μg to be a good inhibitory agent but in our study cloxacillin 300μg or 500μg clearly outweighed the use of boronic acid.
The percentage distribution of Amp C producing E.coli (4.44%) and Klebsiella species (5.19%) in our hospital is quite low compared to other Indian studies which have reports to 6.97% in E.coil and 6.18% in Klebsiellae pneumonia [13], 47.8% E.coli and 13% Klebsiella pneumonia [3], 37.55 E.coli and 24.1% Klebsiella species [11,14,15]. This may be due to the fact that ours is a rural institute catering primarily to poor patients who cannot afford high end antibiotics, therefore limiting their use in the hospital. The absence of this selection pressure is probably responsible for the low resistance in our isolates.
Conclusion
It is not possible to incorporate M3DT as a routine test in any Microbiology laboratory but based on this study results, it is recommended that screening for cefoxitin sensitivity should be incorporated in our routine antibiotic sensitivity testing for all gram negative organisms. The result of the resistant organism should be communicated to the clinicians, so that they may monitor the patients more carefully. It would be prudent to stock the isolates for any further testing till the patient is discharged.
Limitation
The limitation in our study was that, we did not test AmpC production amongst the ESBL producers . One of the study has reported 86.1% (11) of their strains to be both ESBL and AmpC producers.
Acknowledgement
To Dr Meera Sharma, Professor and Head, Department of Microbiology, Post Graduate Institute of medical Sciences, Chandigarh for supplying the AmpC positive control strains.
[1]. RP Ambler, The structure of ß-lactamases.Philos Trans R Soc Lond B Biol Sci. 1980 289:321-31. [Google Scholar]
[2]. K Bush, GA Jacoby, AA Medeiros, A functional classification scheme for betalactamases and its correlation with molecular structure.Antimicrob Agents Chemother. 1995 39:1211-33. [Google Scholar]
[3]. S Arora, M Bal, AmpC β -lactamase producing bacterial isolates from Kolkata hospital.Ind J Med Res. 2005 122:224-33. [Google Scholar]
[4]. CLSI. Clinical and Laboratory Standards Institute. (2011). Performance Standards for Antimicrobial Susceptibility testing: Fifteenth Informational Supplement.Wayne. PaClinical and Laboratory Standards Institute,:M100-S21. [Google Scholar]
[5]. T Yagi, J Wachino, H Kurokawa, S Sujuki, Y Doi K, N Shibata, Practical methods using Boronic acid compound for identification of class C β lactamasesproducing Klebsiella pneumoniae and Escherichia coli.J Clin Microbiol. 2005 43:2551-8. [Google Scholar]
[6]. E Ruppe, P Bidet, C Verdet, G Arlet, E Bingen, First detection of the Ambler class C1 Amp-C β lactamases in Citrobacter frunddii by a new, simple Double –Disk Synergy test.J Clin Microbiol. 2006 44(11):4204-7. [Google Scholar]
[7]. KS Thomson, ME Smith, The new β-lactamases of Gram-negative bacteria at the dawn of the new millennium. Microbiol Infect. 2000 2:1225-35. [Google Scholar]
[8]. V Manchanda, NP Singh, Occurrence and detection of AmpC beta-lactamases among Gram-negative clinical isolates using a modified three-dimensional test at Guru Tegh Bahadur Hospital, Delhi, India.J Antimicrob Chemother. 2003 51:415-8. [Google Scholar]
[9]. KS Thompson, Extended – Spectrum β- Lactamases, Amp C and carbapenemase issues. J clin. Microbiol 2010 48(4):1019-25. [Google Scholar]
[10]. PE Coudron, ES Moland, KS Thomson, Occurrence and detection of AmpC β Lactamases among Escherichia coli, Klebsiellae pneumoniae, and Proteus mirabilis isolates at Veterans Medical Center.J Clin Microbiol. 2000 38:1791-6. [Google Scholar]
[11]. V Hemalatha, M Padma, U Sekar, TM Vinodh, AS Arunkumar, TheDetection of AmpC β lactamases production in Escherichia coli and Klebsiellae by an inhibitor based method.Ind J Med Res. 2007 126:1-3. [Google Scholar]
[12]. GR Trivedi, ST Soni, UV Shah, MM Medeiros, Evaluation of three methods for detection of AmpC β-lactamase in clinical Isolates of Escherichia coli and Klebsiella pneumoniae at a tertiary care hospital.Int J Biol Med Res. 2013 4(3):3475-8. [Google Scholar]
[13]. S Singhal, T Mathur, S Khan, DJ Upadhyay, S Chugh, R Gaind, M Bal, Evaluation of methods for Amp-C β lactamases in gram negative clinical isolates from tertiary care Hospital.Ind. J Med Microbiol. 2005 23(2):120-4. [Google Scholar]
[14]. S Subha, T Renuka, S Ananthan, AmpC β-lactamase producing multidrug resistant strains of Klebsiella spp. & Escherichia coli isolated from children under five in Chennai.Indian J Med Res. 2003 117:13-8. [Google Scholar]
[15]. AK Ratna, I Menon, I Kapur, R Kulkarni, Occurrence & detection of AmpC β-lactamases at a referral hospital in Karnataka.Indian J Med Res. 2003 118:29-32. [Google Scholar]