Hypertension, “The silent killer” is a multifactorial disorder which is asymptomatic and if left untreated leads to lethal complications such as cerebro-vascular accidents and coronary artery disease, failure and sudden cardiac death. Essential hypertension is associated with endothelial dysfunction [1] which is caused mainly by the production of oxygen free radicals that can destroy nitric oxide and impair its beneficial and protective effects on vessel wall causing increase risk of cardiovascular accident [1] In prospective studies it is found that endothelial dysfunction is associated with increased incidence of cardiovascular accidents [1].Hence an antihypertensive agent that reverses endothelial dysfunction could be of value in managing hypertensive patients with endothelial dysfunction e.g. those with diabetes mellitus or hypercholesterolemia and ischaemic heart disease [1].
β blockers are one of the effective drugs for primary and secondary prevention of coronary artery disease. However they are known to cause fatigue, depression, sexual dysfunction, giddiness etc [2] and adverse effect on lipid profile.
Of the available β blocker, Atenolol is the most widely studied in patients of essential hypertension. Hence, in present study Atenolol is used as prototype to compare the efficacy and safety of antihypertensive Nebivolol.
Materials and Methods
This was prospective, double blind randomized, parallel, comparative controlled clinical study conducted in the outpatient department of medicine at tertiary care hospital. Written informed consent was taken from all participants before enrollment and all the tenets of the Declaration of Helsinki were followed during the study. Patients of both sexes between ages of 18-65 years with mild to moderate essential hypertension (diastolic blood pressure 95 -110 mmHg) were included in the study. The patients in this study were newly diagnosed or who have discontinued medication for personal reasons for more than four weeks.
Excluded from the study were patients on other antihypertensives, individuals with angina pectoris or established coronary artery disease, patients with hemodynamically significant valve heart lesions, as well as patients with hepatic, renal impairment and other comorbidities which significantly affect study. Pregnant or lactating females and female patients on child bearing age group not using medically approved contraceptives were also excluded from the study.
The study was conducted in accordance with the Declaration of Helsinki. The study protocol was approved by the institutional ethics committee Government Medical College, Miraj.
Study Schedule and Plan
The patients were enrolled after informed and written consent as per inclusion and exclusion criteria. Current medical history and diagnosis were noted during the first visit. Patients were randomly assigned to receive Atenolol or Nebivolol. After enrollment into the study follow-up was done at baseline than at every two weeks till 12 weeks.
At each visit clinical examination, systolic and diastolic blood pressure of each patient was recorded using mercury sphygmomanometer by auscultation method. The heart rate was also measured at every visit. Biochemical variables assessed were haemogram (HB, TLC), serum creatinine, serum glutamic oxaloacetic transaminase (SGOT), serum glutamic-pyruvic transaminase (SGPT) Total cholesterol, HDL, triglycerides, LDL, urine examination and random blood sugar. Adverse effects if any were recorded in detail at each visit with follow up on same.
Assessment of Efficacy and Safety
The primary efficacy end-point was the change from baseline in sitting diastolic B.P. The other end points included change from baseline in systolic B.P. Target B.P. was 140/90 mmHg.
Those patients who did not show the desired anti hypertensive effect within the stipulated time interval of two weeks were labeled as non responders and referred to the physician for further treatment. Such patients who did not complete full 12 weeks therapy as per study regulations were not included for statistical analysis. Safety was assessed in terms of systemic adverse effects both subjective and objective. Subjective symptoms such as nausea, fatigue were assessed by questioning the patient at each visit. Objective signs were obtained by examining the patient in detail by clinical and biochemical and other examinations. Patient’s global evaluation index was used at the end of treatment. Patient was asked to rate the study drug received as 1- Poor, 2 – Fair, 3 – Good, 4 – Excellent.
Statistical Analysis
Sample size in our study was calculated by using method described by S. Freestone et al., for sample size estimation for short term trails of antihypertensive drugs [6].The power of our study was 90% at 0.05% level of significance. Considering the loss to follow up, total 90 patients were enrolled in the study. Qualitative data such as sex, patient’s habits were analyzed by using chi-square test. Data on adverse effects was analysed using z-test for difference between two proportions. Quantitative data was analysed using z-test for difference between means. p-value < 0.05 was taken as significant. p-value < 0.001 was taken as highly significant, p-value >0.05 was considered insignificant.
Observations and Results
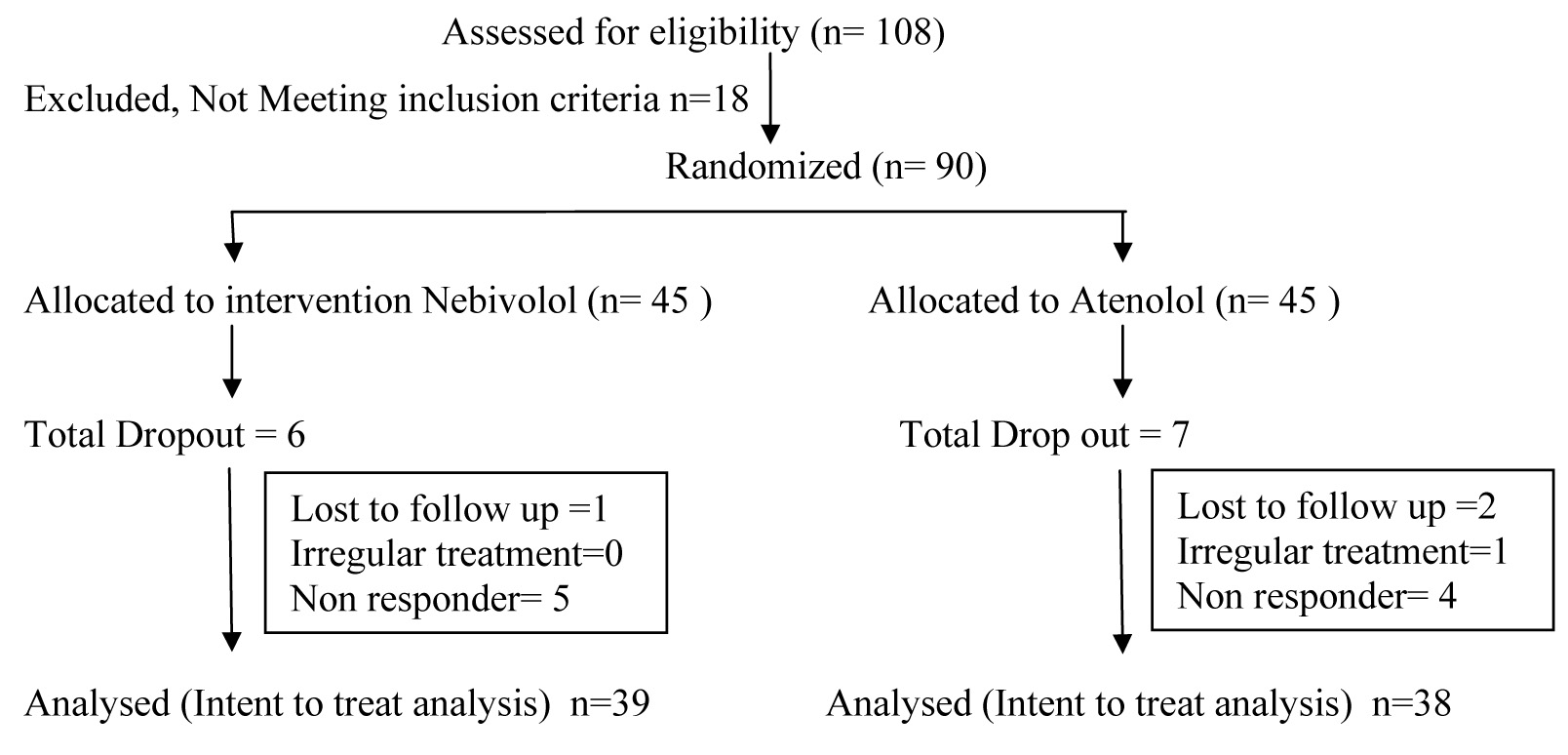
This was prospective, double blind randomized, comparative controlled clinical study. Total 90 patients were enrolled into study as per selection criteria. Patients were randomized to receive Atenolol and Nebivolol with 45 patients in each group. Thity Nine from Nebivolol group and 38 from Atenolol group completed the study as shown in flow chart [Flow Chart].
Two groups were comparable with respect to demographic characteristic [Table/Fig-1].
| Parameters | Nebivolol n=45 | Atenolol n=45 | P-Value |
|---|
| Age (Yrs) | Mean | 51.32± 10.03 | 53 ± 8.61 | p>0.05 |
| Range | 28 – 65 | 37 – 65 | |
| Weight | Mean | 62 ± 8.735 | 63.57 ± 9.97 | p>0.05 |
| Range | 49–82 | 48 - 101 | |
| Sex | Male * | 22 (48.88 %) | 23(51.11 %) | p>0.05 |
| Female * | 23 (51.11 %) | 22(48.88 %) | p>0.05 |
| Habits | Alcohol * | 8(17.77%) | 7(15.55%) | p>0.05 |
| Smoking * | 7(15.55%) | 6(13.33%) | p>0.05 |
- By chi-square test
From [Table/Fig-2], it is seen that nebivolol and Atenolol produced highly significant reduction in both systolic and diastolic blood pressures as compared to baseline value (p<0.001) at second week follow up visit. This reduction in blood pressure was maintained even during 12th week follow up visit.
Effect of nebivolol & atenolol on blood pressure
| S. No | Parameters | Nebivolol (n=39) | Atenolol (n=38) |
|---|
| Systolic B.P. | Diastolic B.P. | Systolic B.P. | Diastolic B.P. |
|---|
| 1 | Baseline | 151.53 ±10.4 | 97.53± 2.4 | 153.63 ± 8.4 | 97.89± 3.47 |
| 2 | After 2 weeks | 140.25± 8.94 | 90.05± 1.86 | 141.57± 5.56 | 90.315± 1.64 |
| 3 | After 4 weeks | 137.02± 8.9 | 88.05± 2.36 | 138.05± 4.79 | 88.57± 2.97 |
| 4 | After 8 weeks | 135.85± 9.26 | 87.43±2.46 | 136.89± 5.0 | 88.05±2.76 |
| 5 | After 12 weeks | 134.25± 8.46 | 86.76± 4.26 | 136.73± 5.08 | 87.84± 4.06 |
| 6 | p-Value | p<0.001 | p<0.001 | p<0.001 | p<0.001 |
After 12 weeks of therapy, the mean reduction in systolic and diastolic blood pressure was not statistically significant when compared between two-treatment groups (p >0.05) [Table/Fig-3] .
Effect of nebivolol compared to atenolol on blood pressure
| S. No | Parameters | Mean reduction in systolic B.P. from baseline | Mean reduction in diastolic B.P. from baseline |
|---|
| Nebivolol | Atenolol | Nebivolol | Atenolol |
|---|
| 1 | After 2 weeks | 11.28±4.3 | 12.06±4.8 | 7.48±2.1 | 7.58±2.3 |
| 2 | After 4 weeks | 14.51±5.2 | 15.58±6.1 | 9.48±1.8 | 9.32±2.05 |
| 3 | After 8 weeks | 15.68±5.8 | 16.74±6.4 | 10.1±2.3 | 9.84±2.42 |
| 4 | After 12 weeks | 17.28±6.2 | 16.9±7.1 | 10.77±2.60 | 10.05±2.83 |
Nebivolol and Atenolol both reduce heart rate significantly (p < 0.001) The mean reduction in heart rate in Nebivolol group was 14.51±4.69 while in Atenolol group was 17.55±5.06 when two groups are compared [Table/Fig-4], the Atenolol reduces heart rate, significantly than Nebivolol (p < 0.05)
Effect of nebivolol and atenolol on Heart rate
| S. No | Parameters | Nebivolol | Atenolol | p-value |
|---|
| 1 | Before treatment | 78.05±5.839 | 76.55±5.33 | p>0.05 |
| 2 | After treatment | 63.53±3.86 | 59.0±3.271 | — |
| 3 | Mean reduction in heart rate | 14.51±4.69 | 17.55±5.06 | p<0.05 |
| 4 | p-Value | p<0.001 | p<0.001 | |
The laboratory investigations like Hb, Total WBC, SGOT, SGPT, Serum creatinine and random Blood sugar random did not show any significant changes after treatment in both groups (p>0.05). The serum cholesterol, Serum triglycerides were slightly increased in atenolol group but it was not statistically significant[Table/Fig-5].
Effect of nebivolol and atenolol on laboratory Investigations
| S. No | Parameters | Nebivolol (5mg) n=39 | Atenolol (50mg) n=38 |
|---|
| Before treatment | After treatment | Before treatment | After treatment |
|---|
| 1 | Hemoglobin | 11.12±1.26 | 11.35±1.21 | 11.05±1.58 | 11.0±1.04 |
| 2 | TLC | 9.11±2.36 | 8.74±1.85 | 8.84±1.45 | 8.71±1.69 |
| 3 | Sr. creatinine | 0.935±0.251 | 0.9±0.171 | 1.02±0.28 | 0.96±0.20 |
| 4 | SGPT | 22.12±7.058 | 20.25±5.217 | 23.1±5.82 | 25.1±6.017 |
| 5 | SGOT | 23.17±7.95 | 21.85±5.55 | 27.2±5.1 | 24.97±6.01 |
| 6 | BSL(R) | 98.2±8.37 | 98.6±7.81 | 97.2±11.02 | 100.15±11.2 |
| 7 | Sr.cholesterol | 213.2±24.17 | 214.9±22.19 | 215.65±20.17 | 222.17±18.31 |
| 8 | Sr.triglyceride | 145.4±23.62 | 146.1±21.48 | 143.75±26.01 | 150.12±20.86 |
| 9 | Sr. HDL | 45.12±8.64 | 46.2±8.18 | 43.55±6.6 | 41.03±5.59 |
| 10 | Sr. LDL | 139.94±23.07 | 141.46±20.45 | 142.92±25.29 | 146.42±22.19 |
The safety analysis was performed on all randomized patients [Table/Fig-6]. The number of patients with adverse effect events was higher in the Atenolol than in the Nebivolol group (12.82% of Nebivolol Vs 36.84% of Atenolol).
Frequency of drug related adverse events
| S.No. | Parameters | Nebivolol | Atenolol | p-value |
|---|
| 1 | Fatigue | 01 | 04 | |
| 2 | Vertigo/dizziness’ | — | 04 | |
| 3 | Hypotension/fainting | 1 | 01 | |
| 4 | Bradycardia | — | 02 | |
| 5 | Headache | 00 | 01 | |
| 6 | Confusion | 00 | 01 | |
| 7 | Constipation | 01 | 00 | |
| 8 | Dysponea | — | — | |
| 9 | Impotence | — | — | |
| 10 | Insomnia | — | — | |
| 11 | Paraesthesia | 01 | 00 | |
| 12 | Skin rash | 00 | 01 | |
| 13 | Sweating | 01 | 00 | |
| TOTAL | 5 (12.82%) | 14 (36.84%) | p<0.05 |
In this case difference achieved statistical significance (p<0.05) showing better tolerability profile of Nebivolol Vs Atonolol.
Majority of patients in Nebivolol group (28.20%, 58.97%) and in Atenolol group (30.84%, 31.57%) rated the therapy as good and excellent while very few (5.128%, 7.69%) in Nebivolol group and Atenolol (13.15%, 18.42%) rated the treatments as poor and fair respectively.
But significantly higher proportion of patients rated Nebivolol as an excellent drug as compared to the proportion of Atenolol treated patients rating the drug as excellent.
Discussion
In the present study, mean reduction in SBP and DBP was slightly higher with Nebivolol as compared to Atenolol,the result was not stastistically significant.Thus both drugs seem to be equally effective in reducing diastolic blood pressure after 12 weeks of treatment. Similarly in studies conducted by Van Nueten et al., [7] and Guide Grassi et al., [8] Nebivolol was compared with Atenolol and placebo in 12 week double blind controlled trials and it was observed that Nebivolol was as effective as Atenolol and decidedly superior to placebo.
At the end of 12 weeks of treatment that both drugs reduce heart rate significantly (p< .001) but the reduction in heart rate was more with Atenolol (p< 0.05) This finding was similar to study conducted by L van Nueten [7] and Otto kamp [9].The reason for this difference could be that Nebivolol reduces blood pressure by β1 receptor blockade as well as reducing peripheral resistance[9] but Nebivolol causes lesser degree of β1 blockade than Atenolol [9].Nebivolol is observed to produce less treatment induced bradycardia as well as tachycardia during exercise.. This may represent an advantage of Nebivolol because changes in heart rate that are too drastic may have adverse effects on patients compliance, particularly during the first period after treatment.
Mean values of lipid profile were unaltered in both groups. This finding is similar to trial conducted by Fogari et al.,[10] in which neither Nebivolol nor Atenolol produced any adverse effects on lipid profile. Nebivolol does not appear to statistically influence plasma lipid metabolism although there have been rare instances of increase in tyiglyceride levels [3]. Atenolol has been found to produce adverse effects on lipid levels over the long as well as the short term in a number of studies. It increases triglyceride level by 18-36% and decrease HDL cholesterol by 6-13%[11].However in light of readings of lipid profile long term studies are required.
Both drugs did not adversely affect the blood sugar level this finding is similar to study conducted by Pessant et al., [12] . But Luc Pioier [13] conducted a study to compare the effects of Nebivolol and Atenolol in 25 ambulatory hypertensive patients with impaired glucose tolerance. His results indicate that insulin sensitivity was not modified significantly by Nebivolol, where as it was reduced by Atenolol by (20%) and 25% by non-selective β blockers like propranolol. The lack of impairment of insulin sensitivity by Nebivolol was probably linked to the extent of β blockade. Many studies have confirmed the correlation between degree of β blockade and insulin sensitivity [13]. Both in the present study and the study conducted by Luc Poirrer et al., [13], Atenolol produced a higher degree of β blockade as indicated by greater reduction in heart rate as compared to Nebivolol. Thus Nebivolol may prove to be beneficial in hypertensive patients with metabolic disorders.
[Table/Fig-6] shows frequency of adverse events. The tolerability of Nebivolol was significantly better than that of Atenolol (p < 0.05) The finding of the present study correlated with that of study conducted by G.Grassi et al., [8] who also found the incidence of adverse events to Nebivolol was significantly lower than that of Atenolol (p < 0.05). In the study conducted by Grassi et al., one patient of Atenolol group but none of Nebivolol group reported to have a dysponea. This adverse reaction was not noted in our study probably due to the exclusion of patients with overt signs of bronchospasm from the study. In other studies [3] the incidence of adverse effects to Nebivolol was comparable to that of placebo treated group. Also Nebivolol scored better than atenolol in terms of Global evaluation which is an important marker of quality of life.
Conclusion
Thus it can be concluded that, for the same antihypertensive effect, Nebivolol was better tolerated than Atenolol.
- By chi-square test