Rare Case of Chest Wall Schwannoma with Destruction of Rib, Masquerading as A Breast Mass
Saikat Datta1, Ananya Pal2, Moumita Maiti3, Anup Kumar Boler4
1Resident, Department of Pathology, N.R.S. Medical College, Kolkata, India.
2Assistant Professor, Department of Pathology, N.R.S. Medical College, Kolkata, India.
3Assistant Professor, Department of Pathology, N.R.S. Medical College, Kolkata, India.
4Assistant Professor, Department of Pathology, N.R.S. Medical College, Kolkata, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Saikat Datta, Doctors’ QTR (Room No-14), N.R.S. Medical College, 25 Dixon Lane, Kolkata- 700014, India.
Phone: 09477011049,
E-mail:drsaikatdatta@gmail.com
Schwannomas are slow growing, benign, nerve sheath tumours of Schwann cell origin. They predominantly involve head, neck and flexor surfaces of upper and lower extremities, while the chest wall is an uncommon location for schwannomas. Schwannomas may rarely cause erosion of adjacent bone. We are reporting a very rare case of a chest wall schwannoma with destruction of rib which occurred in a 35-year-old female patient, which initially presented as a breast mass and was radiologically misinterpreted as a malignant soft tissue tumour.
Verocay bodies, S-100 protein
Case Report
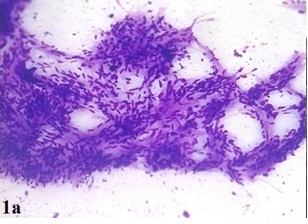
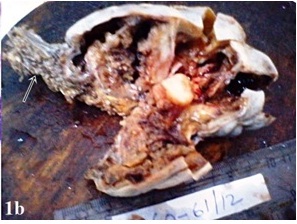
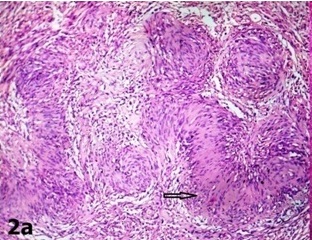
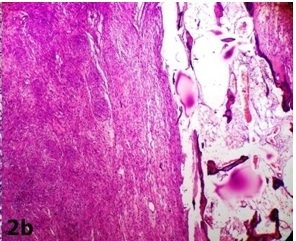
A 35-year-old female presented with the complaints of a slow growing, right breast mass of 1 year and 2 months duration, with occasional dull aching pain in the right chest of 2 months duration. No history of nipple discharge was elicited. On examination, a circumscribed, firm, non tender, globular mass with a maximum dimension of 7 cm was noted in the subareolar region. The mass was fixed to underlying chest wall, but it was unassociated with skin fixity or overlying skin changes. No axillary lymph node was palpable. Given the presentation of a palpable breast mass, the initial imaging evaluation included mammography, which demonstrated only dense breast parenchyma without any breast mass or microcalcification. A contrast enhanced CT scan was performed and it revealed a circumscribed, 7 cm×5 cm×4 cm, diffusely heterogenous, soft tissue mass, probably of chest wall origin, with a osteolytic lesion in right fifth rib. The lesion-lung interface was smooth. CT scan suggested a malignant soft tissue tumour with rib destruction. Fine needle aspiration cytology (FNAC) of the mass was done, which triggered a sharp pain during needling. Smear showed tissue fragments of cohesive cells. The cells had elongated nuclei with pointed ends and indistinct cytoplasm [Table/Fig-1a]. There was a moderate degree of nuclear pleomorphism. A few cells were round to oval, which had mildly hyperchromatic nuclei. Intercellular stroma present in the tissue fragments had a fibrilar appearance. Cytomorphological picture was suggestive of a peripheral nerve sheath tumour, but this could not exclude the possibility of a malignancy. Routine haematological and biochemical investigations done, were within normal limits, except for mild anaemia with an Hb level 10 gm%. The patient underwent wide resection of the mass, with excision of the eroded portion of right fifth rib. She made an uneventful recovery postoperatively. The resected specimen that was sent for histopathological examination, showed a single globular encapsulated, grey-white mass which measured 7 cm×5 cm×4 cm and which encircled a portion of rib [Table/Fig-1b]. Cut surface of the tumour was yellowish, with a few areas of haemorrhage and cystic degeneration. Microscopic examination revealed a tumour with predominantly hypercellular and focal hypocellular areas. The hypercellular areas were composed of compact spindle shaped cells which were arranged in short bundles, interlacing fascicles and forming whorled patterns. The cells showed elongated nuclei with pointed ends, and poorly defined eosinophilic cytoplasm. Hypercellular areas also revealed nuclear palisading, and Verocay body formations of parallel arrays of such palisades with intervening eosinophilic cytoplasm [Table/Fig-2a]. Hypocellular areas were composed of haphazardly arranged spindle shaped cells in the loosely textured matrix, along with scattered inflammatory cells and foamy histiocytes. Hyalinized thick walled blood vessels were seen in these areas. Hypercellular and hypocellular areas kept up with typical Antony A and Antony B areas. One hypocellular area showed few large atypical cells with hyperchromatic nuclei, clumped chromatin and inconspicuous nucleoli, which appeared to be degenerative in nature. Atypical mitotic figures and necrosis were however not seen. Necrosed bony fragments were found to be entrapped within the peripheral portion of tumour mass, which reflected bone destruction caused by the tumour [Table/Fig-2b]. Histopathological findings were consistent with features of conventional schwannomas, which were confirmed by doing a immunohistochemical examination which showed diffuse, strong immunoreactivity for S-100 protein.
FNAC smear showing predominantly spindle cells arranged in fascicles (×100, May-Grunwald-Giemsa)
Encapsulated tumor mass with attached segment of rib showing cortical destruction (arrow)
Verocay body (arrow) composed of parallel arrays of palisaded spindle cells with interveining eosinophilic cytoplasm (×100, Haematoxylin and Eosin)
Bone destruction by the tumor (×100, Haematoxylin and Eosin)
Discussion
Schwannoma, in its classical form, is a benign, non-recurring tumour of adulthood, which occurs most commonly in persons who are between the ages of 20 and 50 years, with no sex predilection [1,2]. The tumour has a propensity to involve head, neck, flexor surfaces of extrimities, posterior mediastinum and retroperitoneum. Chest wall is an uncommon location for schwannomas and they arise from spinal nerve roots or intercostal nerves. Schwannomas can involve bone by three mechanisms: 1. Tumour may arise centrally within a bone, 2. Tumour may arise within nutrient canal, or 3. A soft tissue or a periosteal tumour may cause secondary erosion of bone [3-5]. Our case demonstrated an example of a schwannoma which Secondarily involved bone. This phenomenon is rare and it has been reported to occur in the spinal canal, mandible, zygomatic bone, phalanx of a finger, and the carpus [6-10]. In a review done of 303 cases of solitary benign schwannomas, less than 5% of the tumours were found to be located in anterior, lateral and posterior chest wall and only one patient had a secondary bone erosion [11]. In a review done of magnetic resonance imaging, only one benign schwannoma with cortical ‘pressure erosion’ was noted and this was seen only after its recurrence [12]. After a thorough search of literature, we found only one such case where mild scalloping and erosion of rib were caused by a chest wall schwannoma [13]. In the present case, atypical clinical features like large size of the tumour and presence of bone destruction were misinterpreted by clinician and radiologist as signs of a malignant tumour. This type of misintrepretation of atypical clinical features has been previously reported in the literature, though it was more commonly seen with cellular variants of schwannomas [14]. FNACs of schwannomas are frequently inconclusive and they have potential of being confused with malignant tumours [15]. In our case, due to the presence of atypical cells in cytology smears, possibility of malignant peripheral nerve sheath tumour couldn’t be excluded. Histologically, most important differential diagnosis of a schwannoma is a neurofibroma. A differentiation between these two neoplasms is imperative, because neurofibromas tend to recur frequently and they also have the potential for a malignant transformation. Neurofibromas lack the thick collagenous capsule, Antony A and B patterns, Verocay bodies, which are typical of schwannomas. Immunoreactivity for S-100 protein is observed in about 30-50% cells of a neurofibroma, as opposed to strong uniform reactivity shown by almost 100% cells of a schwannoma. Considering the relatively higher incidence of bone erosion which occurs in cellular schwannomas, it was included in the differential diagnosis of the present case. Cellular schwannomas can be differentiated from conventional ones by their hypercellularities seen almost throughout the tumour, with only focal Antoni B component, rare nuclear palisading and lack of Verocay bodies.Treatment of symptomatic schwannomas consists of marginal resection of tumour with emphasis on identification of the nerve fascicles and excision of the tumour stalk, for thus preventing recurrences while preserving the parent nerve [16]. The nerve of origin is often not identified during surgical excision, as it was in our case. Recurrence is rare, even when the excision is incomplete.
Conclusion
Conventional schwannomas may very rarely present as large soft tissue masses which cause underlying bone destruction and thus lead to an erroneous clinical and radiological diagnoses of malignant soft tissue tumours. Histopathological examination gives a confirmatory diagnosis. Marginal resection appears to be adequate treatment, with very rare incidences of recurrences.
[1]. CF Geschickter, Tumors of the peripheral nervesAm J Cancer 1935 :25-377. [Google Scholar]
[2]. CP Stout, The peripheral manifestations of specific nerve sheath tumor (neurilemoma).Am J Cancer 1935 :24-751. [Google Scholar]
[3]. TG Dahlin, Neurilemmoma of bone: report of 3 cases with review of the literature.Radiology. 1960 75:215-22. [Google Scholar]
[4]. EJ Gordon, Solitary intraosseous neurilemmoma of the tibia: review of intraosseous neurilemmoma and neurofibromaClin Orthop 1976 117:271-82. [Google Scholar]
[5]. Y Park, YW Kim, MH Yang, EJ Kim, DM Ryu, Neurilemmoma of the mandible.Skeletal Radiol 1999 28:536-39. [Google Scholar]
[6]. MR Patel, K Mody, VJ Moradia, Multiple schwannomas of the ulnar nerve: a case reportJ Hand Surg Am. 1996 21:875-76. [Google Scholar]
[7]. M Baranovic, D Macan, EA Begovic, I Luksic, D Brajdic, S Manojlovic, Schwannoma with secondary erosion of mandible: case report with a review of the literature.Dentomaxillofac Radiol 2006 35(6):456-60. [Google Scholar]
[8]. AA Shah, S Latoo, I Ahmad, AH Malik, AP Singh, S Hassan, Schwannoma causing resorption of zygomatic arch.J Oral Maxillofac Pathol 2011 15(1):80-4. [Google Scholar]
[9]. AG Wilson, EP Hofmeister, M Thompson, Recurrent Schwannoma With Bony Erosion of the Distal Middle FingerAm J Orthop 2007 36(3):37-39. [Google Scholar]
[10]. TB Whiston, Neurofibroma eroding carpusJ Bone Joint Surg Br 1953 35:260-61. [Google Scholar]
[11]. TK Das Gupta, RD Brasfield, EW Strong, SI Hajdu, Benign solitary schwannomas (neurilemomas).Cancer. 1969 24(2):355-66. [Google Scholar]
[12]. MA Stull, RP Moser, MJ Kransdorf, GP Bogumill, MC Nelson, Magnetic resonance appearance of peripheral nerve sheath tumors.Skeletal Radiol. 1991 20(1):9-14. [Google Scholar]
[13]. CP Mullan, R Madan, B Trotman-Dickenson, X Qian, FL Jacobson, A Hunsaker, Radiology of Chest Wall MassesAm J Roentgenol 2011 197:460-70. [Google Scholar]
[14]. W White, MH Shiu, MK Rosenblum, RA Erlandson, JM Erlandson, Cellular schwannoma. A clinicopathologic study of 57 patients and 58 tumors.Cancer. 1990 66:1266-75. [Google Scholar]
[15]. LG Dodd, EM Marom, RC Dash, MR Mathews, RE McLendon, Fine needle aspiration cytology of ancient schwannoma.Diagn Cytopathol 1999 20:307-11. [Google Scholar]
[16]. SH Lee, HG Jung, YC Park, HS Kim, Results of neurilemoma treatment: a review of 78 cases.Orthopedics. 2001 10:977-80. [Google Scholar]