Pretibial myxedema (PM) is an infiltrative dermopathy which is seen in grave’s disease. It is also associated with hypothyroidism, but is infrequently seen in Hashimoto’s thyroiditis. Lesions are seen commonly over pretibial region as non-pitting oedema or with a plaque morphology. Heat shock protein (HSP) has been reported to be expressed by fibroblasts present at affected site, which cause lesions of PM. Histopathology differentiates it from other dermatoses. Lesions usually resolve spontaneously, but therapies like potent topical steroids, intralesional steroids, gamma globulin, pentoxifylline, surgery and radiotherapy are indicated. Here, a case of PM with euthyroid Hashimoto’s thyroiditis has been reported, which was proven by histopathology.
Pretibial myxedema, Hashimoto’s thyroiditis, Euthyroid
Case Report
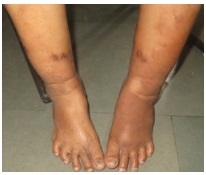
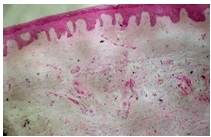
A 63-year-old woman presented with a six month history of a large uritic plaque which was present over both her legs and feet. The lesions had first appeared over her right leg, followed by involvement of left leg. She denied fatigue, heat intolerance, hand tremor, constipation, unexplained weight gain or change in voice. Her past medical history included Type-2 diabetes mellitus, which was currently diet controlled and hypertension, of two year’s duration. Her current medications included an angiotensin receptor blocker. Her physical examination revealed a non-tender, firm, nodular thyroid swelling and exophthalmos. Her cutaneous examination showed a well defined, indurated, erythematous to skin coloured non-tender plaque over both anterolateral aspects of legs. On left side, lesions extended upto middle of the leg and on the right side, just above the ankle, extending to involve bilateral feet, but sparing the toes [Table/Fig-1]. There was no clubbing, hyperhidrosis or hypertrichosis.Thyroid function test showed thyroid-stimulating hormone (TSH)-2.25 mu/L (0.27-4.20 mu/L), free triiodothyronine (T3) -1.54 nmol/L (1.3-3.1 nmol/l), free thyroxine (T4) -93.62 nmol/l (66-181 nmol/l), thyroglobulin antibodies (AbTG) titre- 190 IU/mL(0- 35 IU/mL) and Anti-TPO antibody levels of >1000 IU/mL (0-35iu/ ml). Other haematological and biochemical investigations, including serum creatinine, lipid profile and plasma glucose were within normal limits. Keeping in mind PM, chronic dermatitis and lichen myxedematosus as differentials, a skin biopsy was taken from left leg. Histopathology showed a normal epidermis, collagen fibres in reticular dermis, which were mildly separated by deposits of mucin, and mild perivascular lymphocyte infiltration. These changes were suggestive of PM [Table/Fig-2].
Ultrasound examination of thyroid showed diffusely heterogeous and bulky cystic areas with increased vascularity. Few subcentimetricsized lymph nodes were noted, which were bilateral at level 2 and level 3, which suggested a possibility of Thyroiditis. Fine needle aspiration biopsy taken from thyroid swelling showed oxyphilic cells with round nuclei and moderate to marked anisocytosis, prominent nucleoli and abundant eosinophilic granular cytoplasm. Few multinucleated giant cells with occasional clusters of epitheloid histiocytes were present, which favoured changes of Hashimoto’s thyroiditis. The lesions regressed after 4 sittings, with monthly intralesional triamcinolone acetonide injections, 10 mg/ml, 0.5-1.0 ml per lesion.
Well defined indurated, erythematous to skin colored plaque over both anterolateral aspect of legs, on left side lesions were up to middle of the leg and on the right side just above the ankle, extending to involve bilateral feet but sparing the toes
Histopathological examination shows normal epidermis, with collagen fibers in reticular dermis mildly separated by deposits of mucin and mild perivascular lymphocyte infiltrate. (H&E staining x40)
Discussion
PM is a well-defined cutaneous mucinosis which is characterized by increased amounts of acid glycosaminoglycans in the dermis. It is a rare clinical finding which is often referred to as localized myxedema or thyroid dermopathy. It is an autoimmune manifestation seen in 5–10% of patients with Graves thyrotoxicosis [1] . PM has a gradual onset and it typically develops 12 to 24 months after the diagnosis of thyrotoxicosis. It tends to affect older adults, showing a peak incidence in the sixth decade of life. Women are more frequently affected than men, with female:male ratio of 3.5:1 [2] . It may present as various morphological variants, including nonpitting oedema (43.3%), plaque (27%), nodule (18.5%) and elephantiasis (2.8%) [3] . Though the pretibial area is the classical site of involvement in PM, rarely it has been reported to occur at unusual locations such as the forearms, shoulders, arms, palms, upper back, neck, pinnae and lower part of the abdomen [4] . It occurs over pretibial region in 93.3% of the patients, in pretibial area and feet in 3.9%, and in pretibial area and upper extremities in 1.1%. It is commonly asymptomatic, but rarely pruritic or painful lesions may be present with hyperhidrosis and hypertichosis [2] . The predilection of localization to the pretibial area may result from trauma, with the release of inflammatory cytokines and cells or local hypoxia that results from an arterial or a venous insufficiency [5] . It is occasionally related to euthyroid states and Hashimoto’s thyroiditis [6] . In 1987, the first well-documented association of a localized myxedema with Hashimoto’s thyroiditis was described by Humbert and associates [7] .
PM may also, very rarely, occur in subjects with nonthyrotoxic thyroid disease and even in euthyroid subjects. Lynch et al., [8] reported cases of PM with euthyroidism, but with thyroid gland related clinical or laboratory disorders.
Hashimoto’s thyroiditis usually has clinical manifestations which are similar to those of hypothyroidism, but it has an autoimmune aetiology which is similar to that of Graves’ disease, a common cause of hyperthyroidism. Due to this shared aetiology in both diseases, similar dermatologic and ophthalmologic findings are seen, such as conjunctivitis, diplopia and blurred vision, along with a cutaneous myxedema that can occur in pretibial, scalp, or preradial distributions.
The precise pathogenesis of PM is still enigmatic, but fibroblasts may play a role. TSH and TSH-R antibody binding sites have been reported to be present on the plasma membranes of fibroblasts which are derived from skin of PM patients. It is still unclear as to why the fibroblasts are activated at these sites and the evidence for a site-specific and a generalized fibroblast activation is conflicting. It has been reported that fibroblasts present in the lower limbs are more sensitive to serum fibroblast factor than fibroblasts present at other sites. In contrast, a few studies have concluded that ophthalmopathy and PM are primarily caused by local factors (particularly in the orbit) which are superimposed on a systemic, low- grade connective tissue inflammation. Heat shock proteins (HSP) have been reported to be differently expressed by the fibroblasts present at affected sites, and they play a role in a localized immune process which takes place, which leads to PM [9]. Recently, a thyroid hormone (T3) response gene, ZAK1-4, has been cloned in human fibroblasts, but its role in the pathogenesis of PM remains to be determined [4].
Skin changes which are similar to those seen in PM may develop in patients with simple oedema, due to fluid retention or venous insufficiency, chronic lichenified dermatitis, hypertrophic lichen planus or urticarial phase of bullous pemphigoid. Other rare dermatosis which mimic PM are lichen myxedematosus (papular mucinosis), follicular mucinosis, reticular erythematous mucinosis. All these mucinosis occur over upper limbs, with no thyroid dysfunction and opthalmopathy.
PM requires no treatment, because the lesions may resolve spontaneously. Complete remission of thyroid dermopathy has been reported to occur in 26% of patients, but in an average time of nearly nine years. Various treatment modalities used include steroids (topical, intralesional or under occlusion), compressive stockings, pentoxifylline, gamma globulin, plasmapheresis, surgery and radiotherapy [10]. Local application of a highly potent corticosteroid which is covered with an occlusive dressing is a simple, effective, and a symptomatic treatment for PM. Intralesional triamcinolone therapy is also effective, but side effect of intralesional injections is depressed, irregular and lumpy skin. Experimental use of plasmapheresis has yielded variable results.
Octreotide (an insulin-like growth factor 1 antagonist) injections were reported to be beneficial for treating refractory PM, as per some recent reports [11] .
Conclusion
Our case presented as a rare entity of PM with a diffuse plaque morphology, involving feet and legs but sparing the toes, exopthalamus and Hashimoto’s thyroiditis. Although test for TSH receptor antibodies could not be done, diagnosis of PM and exophthalmos associated Hashimoto’s thyroiditis suggested the existence of an autoimmune mechanism which was responsible for such cutaneous manifestations.
[1]. M Dharmalingam, G Seema, B Khaitan, A Karak, AC Ammini, Plaque form of pretibial myxedema in hypothyroidismIndian J Dermatol Venereol Leprol 2001 67:330-1. [Google Scholar]
[2]. V Fatourechi, Pretibial myxedema: pathophysiology and treatment optionsAm J Clin Dermatol. 2005 6:295-309. [Google Scholar]
[3]. KM Schwartz, V Fatourechi, DD Ahmed, GR Pond, Dermopathy of Graves’ disease (pretibial myxedema): long-term outcomeJ Clin Endocrinol Metab. 2002 87:438-46. [Google Scholar]
[4]. WR Heymann, Cutaneous manifestations of thyroid disease.J Am Acad Dermatol. 1992 26:885-902. [Google Scholar]
[5]. E Senel, AT Güleç, Euthyroid pretibial myxedema and EMO syndromeActa Dermatovenerol Alp Panonica Adriat 2009 18:21-3. [Google Scholar]
[6]. SP Cannavò, F Borgia, M Vaccaro, F Guarneri, E Magliolo, B Guarneri, Pretibial myxoedema associated with Hashimoto’s thyroiditis.J Eur Acad Dermatol Venereol. 2002 16:625-7. [Google Scholar]
[7]. P Humbert, JL Dupond, JP Carbillet, Pretibial myxedema: an overlapping clinical mani-festation of autoimmune thyroid diseaseAm J Med 1987 83:1170-1. [Google Scholar]
[8]. PJ Lynch, JC Maize, JC Sisson, Pretibial myxedema and nonthyrotoxic thyroid disease.Arch Dermatol 1973 107:107-11. [Google Scholar]
[9]. AE Heufelder, BE Wenzel, CA Gorman, RS Bahn, Detection, cellular localization, and modulation of heat shock proteins in cultured fibroblasts from patients with extrathy-roidal manifestations of Graves’ disease.J Clin Endocrinol Metab 1991 73:739-45. [Google Scholar]
[10]. S Veeranna, Kushalappa J Betkerur, Savitha Pretibial myxedema, ophthalmopathy and acropachy in a male patient with Graves’ diseaseIndian J Dermatol Venereol Leprol 2004 70:380-2. [Google Scholar]
[11]. M Shinohara, y Hamasaki, I Katayama, Refractory pretibial myxoedema with response to intralesional insulin-like growth factor 1 antagonist (octreotide): downregulation of hyaluronic acid production by the lesional fibroblastsBr J Dermatol. 2000 143:1083-6. [Google Scholar]