Oral cancer is one of the leading causes of death in India and south east Asia. Squamous cell carcinoma is the most common malignant epithelial neoplasm affecting the oral cavity. In Indian subcontinent oral cancer is mainly due to chewing tobacco and tobacco related products [1].
The orderly progression of the cells through the different phase of cell cycle is precisely governed by a series of proteins called “cyclins”, whose influence effect the binding and activation of the cyclin dependent kinases (CDK). This process is further regulated by phosphorylation, tumor suppressor genes and inhibited by cyclin dependent kinase inhibitors [2]. Dysregulation of the cell cycle mechanism is a critical event in carcinogenesis and it is emerging as a central theme in oral carcinogenesis. The genes involved in cell cycle regulation represent targets of oncogenic abnormalities among which Cyclin D1is most involved [2].
Cyclin D1is a 45 KD (Kilo Dalton) protein. It is a proto-oncogene, encoded by CCND1, located on chromosome 11q13. It is a part of the molecular system that expresess and regulates the cell cycle from G1phase to S phase of cell cycle transition [3]. It was first isolated as PRAD1 (parathyroid adenomatosis 1 gene) oncogene clonally rearranged and over expressed in parathyroid adenomas and was identical to bcl-1 (B-cell lymphoma1 gene) proto oncogene, which is translocated and over expressed in a subset of B-cell neoplasms [4]. Over expression of Cyclin D1 leads to shortening of G1 phase and less dependency on growth factors resulting in abnormal cell proliferation which in turn favours the occurrence of additional genetic lesions [5].
Though tobacco is the most important factor in the causation of the oral squamous cell carcinoma, the expression of cyclin D1 in clinically normal oral mucosa of tobacco habituated individuals has not been studied. The present study is undertaken to fill the void in this important field.
Materials and Methods
The patients and tissue samples for the present study were taken from those attending the Out Patient Department (OPD) of S. Nijalingappa Institute of Dental Sciences and research Gulbarga. The subjects involved belonged to different sections of the society of Northeast Karnataka, India The following inclusion and exclusion criteria were considered for the present study.
Exclusion Criteria
Oral mucosal lesions like, Leukoplakia, Erythroplakia, Oral submucous Fibrosis and patients undergoing radiation and chemotherapy were excluded from the study.
Inclusion Criteria
Tobacco habituated individuals, Non tobacco habituated individual, and Oral cancer patients of both the genders were included in the present study. The present study consisted of three category of subjects.
Group-I, Twelve patients without tobacco chewing or smoking habits with clinically normal oral mucosa served as control cases.
Group-II, Twenty patients with tobacco chewing or smoking habits but with clinically normal oral mucosa.
Group-III, Twenty patients of clinically and Histopathologically confirmed cases of oral squamous cell carcinoma with the habit of tobacco chewing and smoking.
The tissue, representative of lesional area were obtained from all the study group patients by inscisional or excisional biopsy. Informed and written consent was taken from the patients. The use of human tissue samples was approved by the institutional ethical committee.
Methods for Haematoxylin and eosin staining
Formalin fixed, paraffin embedded tissue samples were cut into 5micron sections. The sections were deparaffinised in xylene, rehydrated through graded alcohol. One section was stained for haematoxylin and eosin subsequently mounted under DPX. The Other section was used for immunohistochemical staining.
Immunohistochemical staining-procedure
5 μ sections of formalin fixed, paraffin embedded tissue samples were cut and placed on clean electrostatically charged glass slides.The sections were dried overnight in a 370C incubator. Subsequently sections were dewaxed in xylene. (10 min, 2 changes) and dehydrated in absolute alcohol. The sections were then rehydrated in Propanol (10 min 2 changes) and then washed well in running water. Antigen was retrieved by using 2 litre domestic stainless steel pressure cooker using trisodium citrate buffer. Then the slides were transferred to tris buffered saline (TBS) for 5 minutes twice and subsequently covered with peroxide block (HK111) reagent. The sections are incubated for 10 minutes at room temperature in humid chamber and rinsed well with buffer wash for 5 minute, twice. The tissues were then covered with power block (HK083) reagent and incubated for 5-10 minutes at room temperature in humid chamber. The tissues were then gently blotted. Tissues were covered with primary antibody (Biogenex rabbit monoclonal antibody). Likewise, negative control serum (HK408) was added to the negative control slide. The slides were incubated for 1 hour at room temperature in humid chamber and rinsed well with wash buffer for 5 minute twice. The recommended positive control tissue is thyroid carcinoma tissue. Tissues were covered with super enhancer (HK518) and incubated for 20 minutes at room temperature in humid chamber. The slides were then rinsed well with wash buffer for 5 minute twice. Sections were covered with polymer HRP (HK519) reagent and incubated for 30 minutes, at room temperature in humid chamber. The slides were then rinsed well with wash buffer for 5 minute twice. Tissues were covered with Subsrate Solution (One drop of DAB chromogen (HK124) mixed with 1 ml of substrate buffer (HK520) and incubated for 10 minutes, at room temperature in humid chamber. The slides were rinsed well with wash buffer. The sections were counterstained with haematoxylin, dehydrated, cleared and mounted. The insoluble, permanent brown precipitate formed at the site of antigen (nucleus) was interpreted as positive. The intensity of staining was graded as either negative or positive. The positive intensity of staining is graded as ‘+’ weak, ‘++’ moderate, ‘+++’ intense, absence of staining, ‘-ve’.
Statistical Analysis
Chi - square analysis was used to test the immunohistochemical expression of cyclin D1 with different parameters. Statistical significance was set at p ≤ 0.05.
Results
Six cases of the control group (12 cases) of healthy individuals with normal mucosa did not express cyclin D1, [Table/Fig-1,2] and six cases expressed cyclin D1 in the basal and parabasal layers, of which 2 cases showed weak intensity and 4 cases showed moderate intensity of immunostaining. In tobacco users with clinically normal mucosa, 80% (16/20 cases) expressed cyclin D1. Seven of the twenty cases showed mild dysplasia, of which 6 were positive for cyclin D1, four cases showed moderate intensity and 2 cases showed intense intensity of immunostaining. Six of the twenty cases showed moderate dysplasia, of which five cases were positive for cyclin D1, one case showed weak intensity, two cases showed moderate intensity and two cases showed intense intensity of immunostaining. Cyclin D1 expression in dysplastic lesions is 84.6% (11/20) and a larger percentage of cyclin D1 expression was observed in lower grade dysplasias (53.8 %) than higher grade dysplasias (46.1 %) [Table/Fig-3,4].
APPENDIX – I: Cyclin D1 expression in oral biopsies of normal oral mucosa of individuals without habit of tobacco chewing and smoking
| Sl No. | Age years | Sex | Cyclin D1 expression |
|---|
| 1. S-330/13 | 55 | Male | -ve |
| 2. S-328/13 | 28 | Male | + |
| 3. S-264/13 | 35 | Male | ++ |
| 4. S-258/13 | 50 | Female | ++ |
| 5. S-185/13 | 47 | Male | + |
| 6. S-187/13 | 38 | Male | -ve |
| 7. S-237/13 | 28 | Female | -ve |
| 8. S-256/13 | 45 | Male | ++ |
| 9. S-259/13 | 28 | Male | ++ |
| 10. S-242/13 | 34 | Male | -ve |
| 11. S-254/13 | 48 | Male | -ve |
| 12. S-347/13 | 42 | Male | -ve |
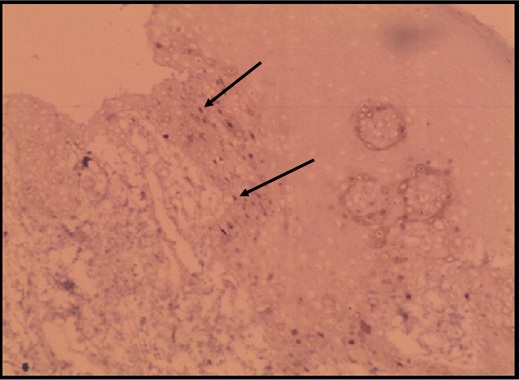
Normal oral epithelium stained weakly with cyclin D1 (100X) Weakly stained cells for cyclin D1 () GROUP – I cases
APPENDIX – II: Cyclin D1 expressions in oral biopsies of tobacco users with clinically normal oral mucosa
| Case No. | Age years | Sex | Tobacco Habit* Smoking(S), Chewing (C) | Dysplasia Grade** | Cyclin D1 expression*** |
|---|
| 1. S-261/13 | 33 | Male | Heavy (c) | ND | +++ |
| 2. S-262/13 | 52 | Male | Heavy (S+C) | Mild | +++ |
| 3. S-265/13 | 28 | Male | Heavy (c) | Moderate | ++ |
| 4. S-245/13 | 49 | Male | Heavy (S+C) | Moderate | +++ |
| 5. S-243/13 | 42 | Male | Heavy (S+C) | Moderate | +++ |
| 6. S-267/13 | 27 | Male | Moderate(C) | ND | + |
| 7. S-251/13 | 23 | Male | Moderate(C) | ND | -ve |
| 8. S-300/13 | 32 | Male | Moderate(S+C) | ND | -ve |
| 9. S-334/13 | 23 | Male | Moderate(C) | ND | +++ |
| 10. S-295/13 | 31 | Male | Moderate(C) | Mild | ++ |
| 11. S-325/13 | 32 | Male | Heavy (C) | ND | ++ |
| 12. S-312/13 | 35 | Male | Heavy (S+C) | Mild | ++ |
| 13. S-332/13 | 24 | Male | Heavy (C) | Mild | ++ |
| 14. S-270/13 | 27 | Male | Heavy (S+C) | Moderate | -ve |
| 15. S-319/13 | 44 | Male | Heavy (C) | Mild | -ve |
| 16. S-292/13 | 45 | Female | Heavy (C) | ND | ++ |
| 17. S-306/13 | 65 | Male | Heavy (S+C) | Moderate | + |
| 18. S-286/13 | 30 | Male | Moderate(S+C) | Mild | +++ |
| 19. S-318/13 | 55 | Male | Heavy (S+C) | Mild | ++ |
| 20. S-272/13 | 45 | Male | Moderate(C) | Moderate | ++ |
Note: * Tobacco use:
(1) Moderate - Chewing tobacco less than 10 packets daily for less than 10 years or smoking less than twenty bidis/cigarettes for less than 10 years.
(2) Heavy - Chewing tobacco more than 10 packets daily for more than 10 years or more than 20 bidis /cigarettes for more than 10 years.
** Dysplasia Grade: Mild, moderate, severe, no dysplasia (ND). ***cyclin D1 expression, intensity of staining. ‘+’ weak, ‘++’ moderate, ‘+++’ intense, absence of staining, ‘-ve’
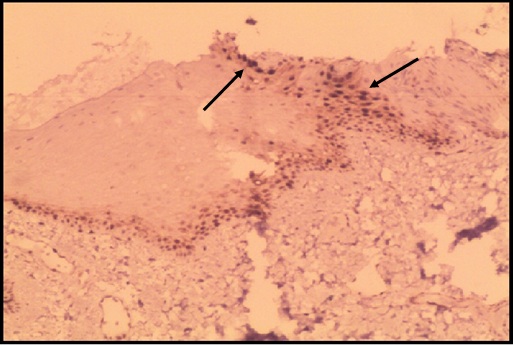
Intensely stained oral epithelium cells of Tobacco chewers with cyclin D1 (100X) Intensely stained cells for cyclin D1 () GROUP – II cases
In oral cancer patients 70% (14/20 cases) showed cyclin D1 expression. Fifteen of the twenty cases were well differentiated, four were moderately differentiated and one was poorly differentiated squamous cell carcinoma. Out of fifteen cases of well differentiated squamous cell carcinomas, four cases showed weak intensity, one case showed moderate intensity and four cases showed intense intensity of immunostaining. Out of four cases of moderately differentiated squamous cell carcinomas, one case showed weak intensity, one case moderate intensity and two case intense intensity of immunostaining with cyclin D1.One case of poorly differentiated squamous cell carcinoma showed moderate intensity of immunostaining [Table/Fig-5,6].
The slides stained by haematoxylin and eosin were classified according to the histological malignancy grading system proposed by Border [18]. Histopathological evaluation was reported by two independent senior oral pathologists.
Discussion
The high incidence of cancers is due to various intrinsic and extrinsic factors. Of the extrinsic factors tobacco is the most important agent. Tobacco smoke is responsible for 80-90 % of the lung cancer cases, [19] and significant percentages of cancers of oral cavity, larynx, oesophagus, pancreas, kidney, bladder and cervix [20].
Cigarette smoke contains at least 3,500 unidentified chemical constituents, nearly 40 % of them being carcinogenic like polycyclic aromatic hydrocarbons (PAH), such as benzo (a) pyrene (Bap), and 4 (N - methyl nitrosoamino) – 1 - (3-pyridil) -1- butanone (NNK), are the important carcinogens. Relatively fewer carcinogens are present in smokeless tobacco. About 30 carcinogens have been identified, the major contributor being nicotine derived nitrosamines (TSNA). Smokeless tobacco is often combined with other reagents including betel leaf, sliced arecanut and powdered slaked lime. These additives used not only enhance the psychotropic effects of nicotine but also make the combination more genotoxic than the tobacco alone [21].
All smokeless tobacco products are buffered to facilitate nicotine absorption through the oral mucosa [22].
The various mechanisms of smokeless tobacco induced carcinogenesis include sister chromatid exchange (SCE), chromosomal aberrations and formation of micro nucleus. The various tobacco products after metabolism in the liver form DNA adducts and cause mutations in vital genes like Rb, P53, K ras etc and also interfere with DNA repair. Haemoglobin adduct formation with tobacco and arecanut extracts like arecoline have been reported [23].
Oncogenes, tumor suppressor genes, viral products and cell cycle related factors are important factors in carcinogenesis. As many as six to ten genetic alterations are involved in the development of head and neck cancer [24]. Escape of cancer cells from the cell cycle machinery reflects a fundamental hallmark of cancer progression and is even emerging as a central theme in oral carcinogenesis [2].
Overexpression of cyclin D1 may lead to shortening of the G1 phase, increased cell proliferation, and reduced dependency on growth factors [25]. This may contribute to disturbance in the normal cell cycle control and mitogenic signaling pathways, enhancing the cell transformation and tumorigenicity [2]. Thus, overexpression of cyclin D1 is thought to provide the tumor cells with a selective growth advantage [26]. The mechanisms underlying cyclin D1 overexpression include gene amplification [27], chromosomal inversion, translocations [5,28,29] transcriptional i.e. upregulation of gene transcription, [30] and posttranscriptional mechanisms [2]. In general, mutations of cyclin D1 gene results in abnormal accumulation of protein, which being much more metabolically stable than the wild protein type, accumulates in the nucleus, reaching the threshold of immunohistochemical detection. Therefore, immunohistochemical demonstration of abnormal presence of cyclin D1 gene product in histological sections of neoplasms may be regarded as an indication of possible mutation of cyclin D1 gene itself.
Over expression of cyclin D1 has been reported in breast carcinomas [31] colon carcinomas, [28] oesophageal carcinomas, [27] head and neck carcinomas, [32] etc and its overexpression has been correlated to advanced clinical stage, [33] poor prognosis, [32],8(8,32) increased risk of recurrence [15] and lymph node metastasis. [6,7,34] A few studies have been carried out to correlate cyclin D1 expression with tobacco use, [9] areca quid chewing and poor prognosis, [10] different degrees of dysplasias and SCC, [12] cisplatin resistance, [13] cyclin dependant kinases [16] in oral squamous cell carcinomas and some have been carried out to correlate the expression of cyclin D1 with histological grading of this neoplasm [1,11,14,17]. However some authors found no clinical correlation [35,36]. Over expression of cyclin D1was seen to be positively correlating with other proliferation markers such as Ki-67, proliferating cell nuclear antigen (PCNA), and other cell cycle regulatory proteins such as CDK4, P21, E2F1 and P53. The above mentioned studies support the role of cyclin D1 as a potential marker of proliferation and oncogenesis.
In this present study cyclin D1 expression is studied by immunohistochemical method using Biogenics rabbit monoclonal antibody in tobacco users with clinically normal mucosa and oral cancer patients habituated to tobacco. The result are compared with immunohistochemically stained sections of twelve control subjects. Cyclin D1 expressions was not observed in six of the control cases. This is consistent with previous studies of total absence of cyclin D1 expressions in normal oral mucosal biopsies [1,12,37]. The absence of cyclin D1 expression in normal individuals could be attributed to the short half life of wild type cyclin D1 protein, hence unable to detect by immunohistochemical methods. Half life of cyclin D1 protein is usually, 24 minutes. [24] Cyclin D1-3, Cyclin E bind with CDK 4 and 6 and CDK 2 respectively, and regulate transition from G1 to S phase. On completion of this task which requires about 24 min, cyclins decline rapidly and hence could not be detected immunohistochemically [25].
Six of the control cases showed cyclin D1 expression in nuclei of cells in the basal and parabasal epithelial layers consistent with the proliferative compartment of stratified squamous epithelium and It indicates that the cells are in G1- S transition phase of cell cycle. This is consistent with previous study of cyclin D1 expression in the basal and parabasal layers of normal mucosa [38–40] [Table/Fig-1,2].
In the present study cyclin D1 expression was found to be positive in 16 (80%) of the 20 cases of tobacco users with clinically normal mucosa. No epithelial dysplasia was observed in 7 cases of tobacco users with clinically normal mucosa. Remaining 13 cases showed dysplasia, out of which 7cases were mildly dysplastic, 6 cases were moderately dysplastic. The expression of cyclin D1 in a majority of tobacco users – smokeless with or without smoking shows the effect of tobacco insult on protooncogenes before clinical alterations are evident and lends credence to the concept that mutation of cyclin D1 protein occur in the early phase of oral carcinogenesis. The expression of cyclin D1 in epithelial dysplasia, though variable from 8.6 % to 82.4 %, [12,41] our results of 84.6 % of a very small sample is quite consistent. Overexpression of cyclin D1 expression is seen in 53.8 % of mild, and 46.1 % of moderate oral epithelial dysplasia, which is consistent with previous studies [40,41] [Table/Fig-2,5].
APPENDIX – III: Cyclin D1 expression in oral mucosal biopsies of histopathologically confirmed cases of oral squamous cell carcinoma
| Case No | Age years | Sex | TNM staging | Histological grade* | Site** | Tobacco Habit*** | Cyclin D1 expression*** |
|---|
| 1. S-299/13 | 65 | Male | T4N0M0 (IV) | WD | P | Moderate (C+S) | -ve |
| 2. S-225/13 | 70 | Female | T4N0M0 (IV) | MD | P | Moderate (C) | +++ |
| 3. S-230/13 | 38 | Male | T2N0M0 (II) | WD | MBS | Heavy (C) | -ve |
| 4. S-110/13 | 58 | Male | T2N0M0 (II) | WD | MBS | Heavy (C) | + |
| 5. S-27/12 | 50 | Male | T2N0M0 (II) | WD | T | Heavy (C) Moderate (S) | +++ |
| 6. S-12/12 | 45 | Female | T2NOM0 (II) | PD | BM | Heavy (C) | ++ |
| 7. S-44/13 | 37 | Male | T3NOMO (III) | WD | MBS | Moderate (C) | +++ |
| 8. S-196/12 | 60 | Male | T1N0M0 (I) | WD | P | Moderate (C) Heavy (S) | +++ |
| 9. S-286/12 | 30 | Female | T2NoMO (II) | MD | LAM | Heavy (C) | +++ |
| 10. S-127/11 | 60 | Female | T2N0M0 (II) | WD | LMAG | Moderate (C) Heavy (S) | + |
| 11. S-162/11 | 32 | Female | T2N0M0 (II) | WD | T | Moderate (C) | -ve |
| 12. S-107/11 | 39 | Male | T4N0M0 (IV) | WD | BM | Moderate (C) | +++ |
| 13. S-47/13 | 45 | Male | T2N1M0 (II) | WD | LAM | Heavy (C) | -ve |
| 14. S-92/11 | 50 | Female | T2N0MO (II) | WD | BM | Moderate (C+S) | + |
| 15. S-91/11 | 50 | Male | T3N0M0 (III) | WD | BM | Moderate (C+S) | -ve |
| 16. S-74/11 | 50 | Female | T2N0M0 (II) | WD | RMA | Heavy (C) | ++ |
| 17. S-54/11 | 54 | Male | T2NOM0 (II) | WD | BM | Moderate (C) | -ve |
| 18. S-74/13 | 45 | Male | T1N0M0 (I) | WD | T | Moderate (C+S) | + |
| 19. S-59/13 | 58 | Female | T2N1M0 (III) | MD | T | Moderate (C) | ++ |
| 20. S-60/13 | 62 | Male | T1N0M0 (I) | MD | LLM | Heavy (c+S) | + |
Note: * Histological grade: Moderately differentiated (MD), Well differentiated (WD), Poorly differentiated (PD). (Broders grading).
** Site: Buccal Mucosa (BM), Tongue (T), Palate (P), Lower Alveolar Mucosa (LAM), Lower Lip (LL), Mandibular buccal sulcus(MBS), Lower marginal and attached gingival(LMAG), Retromolar area(RMA), Lower labial mucosa(LLM). *** Tobacco Habit & Cyclin D1 expression as in Appendix - II.
The progression of the disease in the present study increased from early age to older age in the group- III cases. In the group -II cases, 55 percentage of cases were positive for cyclin D1 (11/20 cases), in the early age group i.e second to third decade of life is indicative of the fact that the individuals were susceptible to the genotoxic effects of tobacco and its related products, leading to the conclusion that the disease process being active at cellular and molecular level even before the apparent clinical changes are manifested. In the control group cases the expression was observed in all the age group patients in basal and supra basal layers, indicative of active cell proliferation in these cell layers.
In the present study expression of Cyclin D1is observed in 14 of the 20 cases (70%) of histopathological confirmed cases of oral squamous cell carcinoma cases with tobacco habituation. Though cyclin D1 expression was observed by all workers in oral cancer biopsies, with the expression varying from 32% to 88.5%, the results of 70 % cyclin D1 expression in this study is consistent with previous studies [12,32,39,42]. The higher expression of cyclin D1 can be attributed to the intensity, duration and form of tobacco used and the peculiar Indian habit of smokeless tobacco use. All the patients in this study were tobacco users of smoking and smokeless tobacco along with betel leaf and arecanut, of at least 10 years duration and above. Thus the high incidence could be explained due to combined effect of tobacco smoke, chewing tobacco and arecanut which is proved to be genotoxic [Table/Fig-5,6].
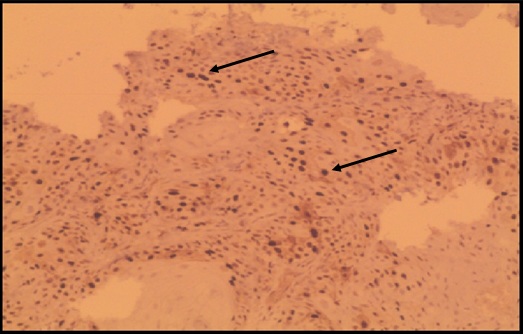
Intensely stained squamous cell carcinoma with cyclin D1 (100X) Intensely stained cells for Cyclin D1(↑) GROUP . III cases
Most of the well differentiated squamous cell carcinomas showed cyclin D1 expression largely confined to the periphery of tumour nests consistent with the basal cells of tumor nests.
The percentage of cyclin D1 expression in carcinomas (70%) was reduced in comparison with the percentage of dysplastic lesions (84.6%) and we also found a larger percentage of cyclin D1 expression in lower grade dysplasias (53.8%) than higher grade dysplasias (46.1%) and squamous cell carcinomas (70%), which may be indicative of increased traverse through the cell cycle, occuring early in tumor progression [41].
With regard to cyclin D1 expression and grading in oral squamous cell carcinoma cyclin D1 expression increased with decrease in differentiation [1,11]. Since four cases, in this study belonged to moderately differentiated squamous cell carcinoma, 15 belonged to well differentiated squamous cell carcinoma and only one belonged to poorly differentiated squamous cell carcinoma, association between cyclin D1 expression and histological grades is not found statistically.
Over expression of cyclin D1 was significantly associated with regional lymph node metastases and advanced tumor stage [33]. Since three cases belonged to stage I, stage III and stage IV each, 11 cases belonged to stage II the association of cyclin D1 to different stages could also not be found statistically.
Though, there have been many studies of cyclin D1 expression on oral biopsies of squamous cell carcinoma, [3,7–16] the expression of cyclin D1 in clinically normal oral mucosa of tobacco habituated individuals has not been studied.
However, a study of a large samples of oral cancer cases and tobacco users with clinically normal mucosa cases by biopsies is needed to corroborate our findings.
Note: * Tobacco use:
(1) Moderate - Chewing tobacco less than 10 packets daily for less than 10 years or smoking less than twenty bidis/cigarettes for less than 10 years.
(2) Heavy - Chewing tobacco more than 10 packets daily for more than 10 years or more than 20 bidis /cigarettes for more than 10 years.
** Dysplasia Grade: Mild, moderate, severe, no dysplasia (ND). ***cyclin D1 expression, intensity of staining. ‘+’ weak, ‘++’ moderate, ‘+++’ intense, absence of staining, ‘-ve’
Note: * Histological grade: Moderately differentiated (MD), Well differentiated (WD), Poorly differentiated (PD). (Broders grading).
** Site: Buccal Mucosa (BM), Tongue (T), Palate (P), Lower Alveolar Mucosa (LAM), Lower Lip (LL), Mandibular buccal sulcus(MBS), Lower marginal and attached gingival(LMAG), Retromolar area(RMA), Lower labial mucosa(LLM). *** Tobacco Habit & Cyclin D1 expression as in Appendix - II.