Eye is a unique organ that is virtually impermeable to most of the environmental agents. However, in some circumstances, infectious agents gain access to the posterior segment of the eye through one of the three routes: as a consequence of an intraocular surgery [1], following a penetrating injury of the globe and through a haematogeous spread of bacteria to the eye from a distant anatomical site. The infection of eye leads to conjunctivitis, corneal ulcers and endophthalmitis, which may lead to blindness of the eye. Suppurative keratitis can cause corneal opacity and perforation, which can lead to severe visual loss and blindness. Staphylococcus spp. continues to be the predominant cause of bacterial keratitis, but the causative organism may vary geographically. Coagulase negative Staphylococci (CoNS) are common inhabitants of human skin and mucous membranes. S. epidermidis species is found most commonly as a member of normal flora. The pathogenic potential of CoNS has received only little attention. More than 30 species of CoNS have been recognized, but only a few have been commonly incriminated in human infections. Infections caused by CoNS, can be caused by either community or hospital acquired isolates [2]. Skin of patients and health care workers, medical equipment, clothing of personnel and environment surfaces can be sources of antibiotic resistant S. epidermidis strains [3]. Skin contact affords an easier transmission between hosts [4]. Colonization pressure makes an impact on both the risk for a cross transmission between patients and the risk for patients in acquiring a nosocomial infection [5]. Thus, coagulase negative Staphylococci are becoming a group of microorganisms that can be increasingly implicated as a cause of significant infection. Many clinical microbiology laboratories do not identify CoNS to the species level, even when these microorganisms are detected in blood or cerebrospinal fluid. However, as the pathogenic significance of CoNS increases, it may become more important to learn more about the epidemiology and pathogenic potential of individual species. This may be particularly important with regards to blood culture isolates, since it is often difficult to determine the clinical significance of an individual isolate. The virulence factors of CoNS which cause corneal infections may be attributed to slime production, haemagglutination and extracellular enzymes lipase and protease. Owing to these circumstances and in order to have an in-depth understanding of CoNS, the present study was carried out, considering the importance of coagulase-negative Staphylococci as a causal agent of ocular infections.
Materials and Methods
Collection and Processing of Ocular Samples
Infected corneal specimens were collected from the patients who attended a tertiary care eye hospital in Coimbatore, Tamilnadu state, India. Patients with corneal ulcerations which lasted for more than 3 weeks were excluded. Patients who were suspected of having fungal or viral infections, or Acanthamoeba keratitis were also excluded. Corneal scrapings were collected from cases who were suspected of having keratitis, after a thorough examination was done by an ophthalmologist.
The specimens were collected by using a sterile Kimura’s spatula aseptically, under slit lamp illumination, after administrating a local anaesthetic such as lignocaine. Specimens were collected from cases of conjunctivitis and endophthalmitis as conjunctival swabs and vitreous taps respectively and they were processed. The scrapped out materials were directly streaked onto solid media such as blood agar and brain heart infusion broth and the plates/tubes were incubated at 37°C for 24 hours. The scrapings were also placed onto clean, scratch free labeled glass slides for Gram staining. The colonies, after incubation, were further streaked onto nutrient agar, chocolate agar and mannitol salt agar and the plates were incubated at 37°C for 24 hours.
Characterization of CoNS
Slide coagulase test and tube coagulase test were performed initially, to characterize CoNS. Catalase test, oxidase test, indole production test, methyl red test, Voges-Proskauer test, citrate utilization test, urease test, nitrate reduction test and sugar fermentation test (trehalose, sucrose and lactose) were employed for the identification of the isolates [6].
Screening of virulence factors
For the screening of lipase and protease activities, sterile tributyrin agar base with tributyrin oil and skim milk agar respectively, were inoculated with the test organism and the plates were incubated at 37°C for 24 hours and observed for zones of clearance [7]. For the investigation of slime production, the test isolates were inoculated onto sterile Congo red agar plates, which were incubated at 37° C for 24–48 hours. Following their incubations, an observation was done for black coloured colonies [8]. For haemagglutination test, to a 1% sheep erythrocyte suspension which was prepared in phosphate - buffered saline (PBS), bovine serum albumin was added to a concentration of 0.1%, in order to prevent nonspecific agglutination. Test isolates, after an overnight incubation at 37°C in Muller Hinton broth, were harvested by centrifugation and they were washed once with PBS. The bacterial suspensions were adjusted to McFarland’s standard of 3.0, which correlated with approximately 108 bacteria per ml. The bacterial suspensions were diluted to 1:10 in PBS. The haemagglutination assay which used a twofold serial dilution of the 10% bacterial suspension was performed in 96-well (U-shaped) microtitre plates, to give a total volume of 50 μl per well. Then, 50 μl of the 1% erythrocyte suspension was added to each well. The plates were sealed with cellophane, shaken to ensure even mixing of the bacteria and erythrocytes, and incubated at room temperature for 2 hours. Haemagglutination was rated on a scale of 0 to + (0 haemagglutination was recorded when a compact pellet of erythrocytes was observed, and + was recorded when diffuse haemagglutination was observed) [9].
Speciation of the Isolates
The test isolates of CoNS were subjected to various tests viz., phosphatase, arginine hydrolysis, sugar (trehalose and maltose) fermentation and novobiocin susceptibility, to identify them till species level. For phosphatase test, one loopful of the 24 hours isolates obtained from the phosphate agar was touched onto a filter paper which was saturated with 1N NaOH. Formation of a pink colour indicated a positive result. For arginine hydrolysis test, colour change of inoculated arginine broth from yellow to purple, after 24 to 48 hours, indicated a positive result. For sugar fermentation test, sterile peptone broth which contained phenol red and respective fermentable sugars viz., trehalose and maltose was prepared. The tubes were inoculated with the isolates, they were incubated at 37°C for 24–28 hours, and observed for acid production. Novobiocin susceptibility of the isolates was done on sterile Muller Hinton agar plates by using standard procedures [6].
Antibiotic Susceptibility Testing
Antibiotic susceptibility testing was performed for all the confirmed strains of CoNS on Muller Hinton agar, in accordance with the procedure which was outlined by Clinical and Laboratory Standards Institute [10]. Eighteen different antibiotics (Hi-Media) which belonged to ten groups, at varying concentrations, were selected for antibiogram analyses.
Results
A total of 260 samples were collected from patients who attended the diagnostic laboratory of a tertiary care eye hospital in Coimbatore, Tamilnadu, during the study period (October, 2011 to August, 2012). After 24 hours of incubation, colony morphology were noted on different culture media. In nutrient agar, an observation was made for colonies that formed clusters, appeared grey or white in colour, circular, with or without slight pigmentation, 2-4 mm in diameter and convex, with shining surfaces. On mannitol salt agar, the isolates that failed to ferment mannitol and those that formed small orange colonies which were surrounded by red or purple medium, were selected for further study. In blood agar and chocolate agar, an examination for gamma haemolysis was made.
Out of 260 samples which were processed, a total of 146 isolates of Gram positive cocci in clusters [Table/Fig-1] were identified, among which, 100 were confirmed as coagulase negative Staphylococci, after they failed to coagulate the plasma in both slide and tube coagulase tests. The isolates of CoNS were designated as CoNS 1- CoNS 100.
Microscopic view of CoNS after Gram’s staining (100X)
Of the 100 patients who had been carrying CoNS due to various ophthalmic complications, 65 and 35 were found to be males and females respectively. Likewise, a majority (45%) of the target patients who were included in the study were found to be between 70 and 79 years of age, followed by those who were 80-89 years of age (25%) and the remaining falling between the age group of 10 and 69 years.
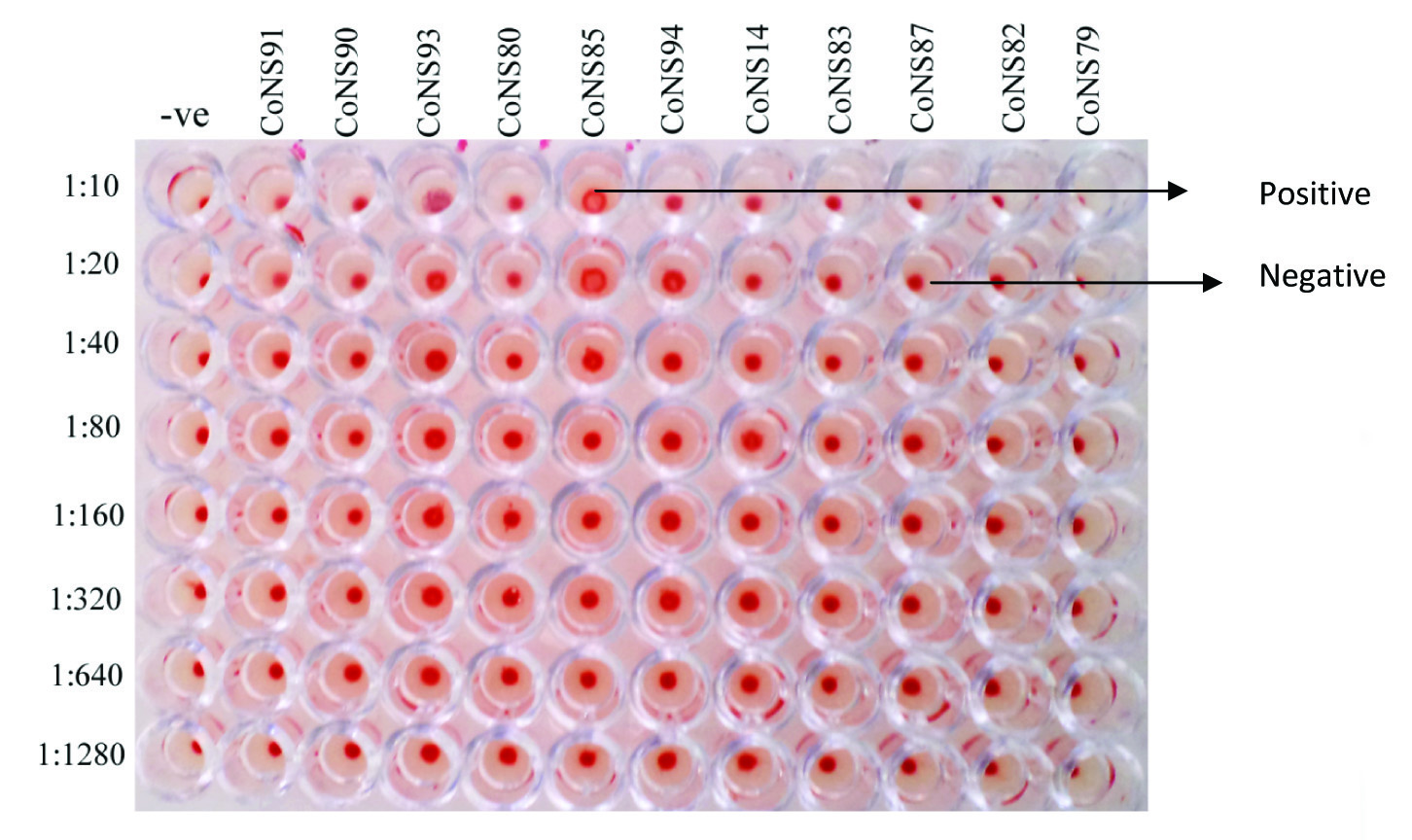
Precisely, 100 % and 38 % of the isolates were positive for lipase and protease productions. Congo red agar method showed that 63% of the isolates were positive for slime production [Table/Fig-2]. Out of 100 isolates, only 30 isolates viz., 12 from endophthalmitis cases, 5 from corneal ulcer cases and 13 from preoperative cases were subjected to the haemagglutination test. Of the 30 cases, 4 isolates of CoNS showed a positive result ie. Complete haemagglutination [Table/Fig-3 & 4].
CoNS isolated from ocular complications
| S. No | Cases | No. of CoNS isolates |
|---|
| 1. | Endophthalmitis | 12 |
| 2. | Corneal ulcer | 05 |
| 3. | Pre operative cases | 83 |
| Total | 100 |
Virulence pattern of CoNS
| S.No | Virulence factors | Percentage of isolates showing positive results (%) |
|---|
| 1 | Lipase (n=100) | 100 |
| 2 | Protease (n=100) | 38 |
| 3 | Slime production (n=100) | 63 |
| 4 | Haemagglutination (n=30) | 13 |
Microtitre plate showing haemagglutination by CoNS
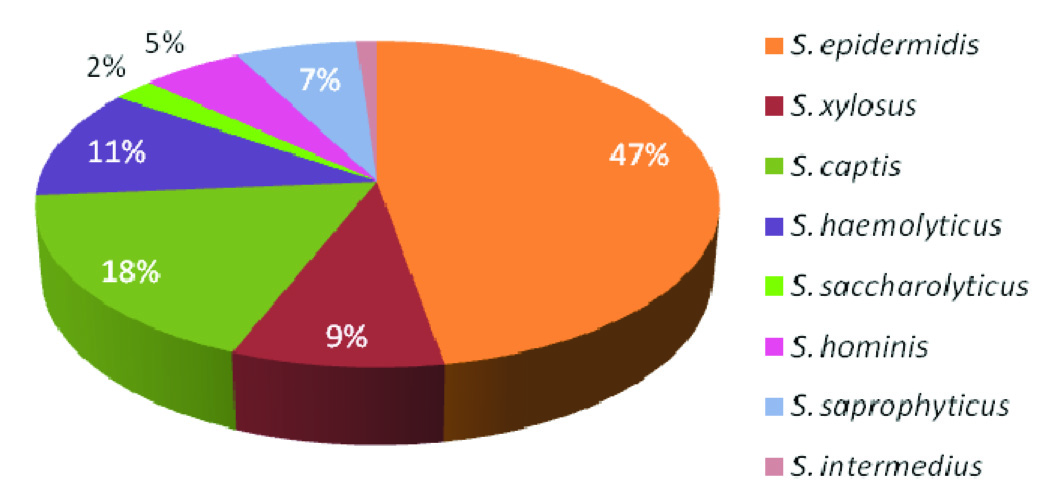
Of the 100 isolates of CoNS, 43% were identified as S. epidermidis. The other isolates were identified as S. xylosus (n=8), S. captis (n=16), S. haemolyticus (n=10), S. saccharolyticus (n=2), S. hominis (n=5), S. saprophyticus (n=6) and S. intermedius (n=1) [Table/Fig-5].
Speciation of test isolates of CoNS based on Cowan and Steel (1995)
The antibiotic susceptibility pattern revealed that all the isolates were sensitive to cefazolin and linczolid. Further, 89%, 80%, 80% and 86% of the isolates were sensitive to amikacin, gentamycin, ciprofloxacin and novobiocin respectively. It was found that all the isolates were resistant to bacitracin and nalidixic acid, whereas 90% of the isolates were resistant to cefatoxime and oxacillin and 4 % of the isolates were resistant to methicillin. It was also noted that 5% of the isolates were intermediately susceptible to amikacin [Table/Fig-6].
Antibiotic susceptibility pattern of CoNS
| Antimicrobial agents | Disc content (μg) | Sensitive (%) | Intermediately susceptible (%) | Resistant (%) |
|---|
| Amikacin | 30 | 89 | 5 | 6 |
| Gentamycin | 10 | 80 | - | 20 |
| Cephotaxime | 30 | 10 | - | 90 |
| Cefazolin | 30 | 100 | - | - |
| Linezolid | 30 | 100 | - | - |
| Ciprofloxacin | 30 | 80 | - | 20 |
| Nalidixic acid | 30 | 10 | - | 90 |
| Vancomycin | 30 | 72 | - | 28 |
| Oxacillin | 1 | 10 | - | 90 |
| Methicillin | 2 | 96 | - | 4 |
| Erythromycin | 10 | 55 | - | 45 |
| Bacitracin | 10 | - | - | 100 |
| Novobiocin | 30 | 86 | - | 14 |
Discussion
CoNS are currently the most common organisms which are isolated from cases of postoperative endophthalmitis. Species identification and investigation of virulence factors will improve our knowledge on the roles which are played in clinical diseases by this group of bacteria. In the present investigation, from 260 ocular samples, a total of 100 CoNS were isolated. In a study which was conducted by Bharathi et al., [11] the predominant bacterial species which was isolated was Streptococcus pneumoniae, followed by Pseudomonas aeruginosa and S. epidermidis. CoNS that made up to 43% of the total Gram positive cocci were also found to be a predominant pathogen, as was reported in a study from Ghana [12]. According to Gopinathan et al., [13], a male preponderance was observed in case of bacterial keratitis. Though both sexes develop corneal ulcers, more commonly, in the middle decades of life, a significant male preponderance was reported; including those seen in children and elderly patients. In the current study, 45% of the target patients were found to be between 70 and 79 years of age.
Unlike their coagulase-positive counterpart, Staphylococcus aureus, CoNS produce few virulence patterns and they normally refrain from invading tissues. Presence of lipase enzyme was demonstrated in Staphylococci which were associated with infections. In this study, all the isolates were positive for lipase activity. Similar results were obtained by Saising et al., [7]. Lipolytic enzymes which were produced by CoNS demonstrated that lipase enzymes were important for the organisms, for their colonization and growth within the lipid-rich environment of the skin [14]. Burkhart et al., [15] correlated the lipase producing CoNS with virulence, where, of 64 coagulase-positive isolates, only 42 isolates (65.6%) were found to be lipase positive.
A secreted bacterial protease may also act as an exotoxin, and be an example of a virulence factor in bacterial pathogenesis. Staphylococci, in particular, S. aureus, are known to produce several extracellular proteases, including serine-, cysteine- and metalloenzymes. Their insensitivities to most of the human plasma protease inhibitors and, even more, their ability to inactivate some of these, make the proteases potentially harmful. A tight, cell density-dependent control of proteolytic activity expression, which is similar to that of the well-defined virulence determinants, further suggests the role of Staphylococcal proteases in the infection process [16]. According to Saising et al., [7] coagulase-negative Staphylococcal isolates produced both lipase and protease enzymes more frequently than the coagulase-positive group.
Slime is believed to make the microorganisms more resistant to administered antibiotics and to host defence mechanisms [17]. Slime production may reflect the organism’s capacity to adhere to specific host tissues and thereby, to produce invasive microcolonies and hence, slime production is an important virulent factor. It was also shown that comparatively higher numbers of slime producing isolates of S. epidermidis obtained from the keratitic lesions were adherent to artificial surfaces, as compared to the commensal isolates from the eye [18]. In the same study, Nayak et al., [18] obtained 39.4% of slime producing isolates of S. epidermidis. However, our study showed that 63% of the isolates were positive for slime production. The haemagglutin of CoNS appears to be a protein that interacts specifically with oligosaccharides on the surface of erythrocytes [19]. Further studies are required to elucidate the nature of the S. epidermidis haemagglutinin.
In our study, S. epidermidis was found to be the most frequent isolate (43%), followed by S. captis (16%), S. haemolyticus (10%), etc. This study’s findings correlated with those of the studies of Singh and Banerjee [20], where they identified S. epidermidis (40%) as the most frequently obtained clinical isolate, followed by S. saprophyticus (14%), S. haemolyticus (12%), S. hominis (6%) and S. lugdunensis (6%). Manikandan et al., [21] showed that S. epidermidis was the predominant species which was obtained, both clinically and saprophytically. The biochemical tests were used as the basis for speciation, as other advanced methods such as automated systems and sequencing were very expensive. Faghri and Razavi [22] determined the presence of the genes, icaADB and icaD in all the slime producing isolates of CoNS obtained from ocular wound infections, which had occurred after laser refractive surgeries. They further characterized them to species level by using ID32 Staph. All 22 isolates obtained from ocular infections were presumptively identified as one of the species of CoNS group. Makki et al., [23] used the analytical profiling index identification system and identified S. haemolyticus, S. xylosus, S. capitis and S. hominis from ocular infections.
In the antibiotic susceptibility analysis, it was found that most of the isolates were sensitive to vancomycin, amikacin and linozolid and resistant to cefatoxime, oxacillin, bacitracin and nalidixic acid. Oxacillin resistant CoNS were reported from different parts of Europe, whose incidences were between 70% and 80%, and similar reports were also available from the United States, Canada and Latin America [24–26]. CoNS showed maximum resistance to ampicillin (89%), followed by cefotaxime (59%), as was reported by Goyal et al., [27], to which our data was compatible. Resistance to erythromycin and vancomycin, seen in our data was higher than those which were reported by other studies [27–31]. In the study conducted by Pinna et al., [32] CoNS isolated from patients with corneal and external infections showed highly variable patterns of antibiotic susceptibility, where only 8 (15%) strains were found to be sensitive to all antibiotics and most of the isolates were penicillin and tetracycline resistant, whereas resistance to ciprofloxacin and teicoplanin was found in 4 (7%) and 1 (2%) isolates, respectively. Knowledge on the susceptibility or resistance pattern of CoNS is a prerequisite for rational use of antibiotics.
Conclusion
Coagulase negative Staphylococci (CoNS), which were previously dismissed as contaminants, are now emerging as important potential pathogens, due to an increase in number of severely debilitated patients and increased use of implants in hospitals. Unlike their coagulase-positive counterpart, Staphylococcus aureus, CoNS produce few virulence patterns and they normally refrain from invading tissues. The virulence factors of CoNS which cause corneal infections may be attributed to slime production, haemagglutination and extracellular enzymes viz., lipase and protease. Owing to circumstances and in order to have an in depth understanding on CoNS, a study was conducted, considering the importance of coagulase-negative Staphylococci as a causal agent of ocular infections.