Alpha-1 Antitrypsin, a Diagnostic and Prognostic Marker of Vernal Keratoconjunctivitis
Akif Ahsan1, Khushtar A Salman2, Sana Alam3, Anwar H Siddiqui4, Syed Shariq Naeem5, Aquil Ahmad6, Iqbal M Khan7
1 Junior Resident, Department of Biochemistry, Jawaharlal Nehru Medical College, A.M.U., Aligarh, UP, India.
2 Associate Professor, Department of Biochemistry, Jawaharlal Nehru Medical College, A.M.U., Aligarh, UP, India.
3 Junior Resident, Department of Biochemistry, Jawaharlal Nehru Medical College, A.M.U., Aligarh, UP, India.
4 Senior Resident, Department of Physiology, Jawaharlal Nehru Medical College, A.M.U., Aligarh, UP, India.
5 Senior Resident, Department of Pharmacology, Jawaharlal Nehru Medical College, A.M.U., Aligarh, UP, India.
6 Senior Resident, Department of Physiology, Jawaharlal Nehru Medical College, A.M.U., Aligarh, UP, India.
7 Assistant Professor, Department of Preventive and Social Medicine, Jawaharlal Nehru Medical College, A.M.U., Aligarh, UP, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Akif Ahsan, Junior Resident, Department of Biochemistry, JNMC, AMU, Aligarh–202002, India.
Phone: +91-8755547610,
E-mail: akif12345@gmail.com
Introduction: A major chunk of ocular allergies in humans involve the conjunctiva, of which Vernal Keratoconjunctivitis (VKC) appears to be more common. VKC, a chronic allergic conjunctivitis, frequently affects young males and is characterized by intense inflammation of the limbal and/or tarsal conjunctiva. The etiology and immuno-pathogenesis of VKC still remain unclear.
Alpha-1 antitrypsin (AAT), a member of serine proteinase inhibitor (SERPIN) superfamily, is an acute phase protein whose concentration in blood increases in response to inflammation. AAT deficiency is one of the many factors that may be involved in several abnormalities such as liver disease, emphysema, inflammatory joint diseases and inflammatory eye diseases. In the present study, the role played by this protein in VKC was analyzed in a selective case/control study to assess its diagnostic and prognostic value.
Materials and Methods: The case control study included 50 patients of VKC reporting to Ophthalmology out patient department (OPD). Age and sex matched 40 healthy subjects served as control. Serum AAT level of both the cases and controls were evaluated and compared. Moreover the serum AAT levels of the patients at presentation were compared with their serum AAT level after three weeks post treatment.
Result: Levels of AAT in the serum of VKC patients at presentation (2.80 ± 0.42 mg/ml) were significantly higher as compared to controls (2.31 ± 0.21 mg/ml) whereas no significant difference was observed between the serum level of post treatment VKC patients (2.48 ± 0.26 mg/ml) and controls.
Conclusion: AAT is a potent acute phase protein whose concentration rises significantly in VKC, irrespective of the age and sex of the patient. Moreover, the serum level of AAT declined significantly post treatment; therefore it might be used as a prognostic marker.
Alpha-1 antitrypsin, Vernal keratoconjunctivitis, Trypsin inhibitory activity
Introduction
Ocular allergy and uveitis are varied groups of inflammatory eye disorders characterized by complex and as yet ill-defined pathogeneses. Vernal keratoconjunctivitis (VKC), a type of chronic allergic conjunctivitis [1], is a bilateral condition frequently seen in children and young males, characterized by intense recurrent interstitial inflammation of the superior and limbal palpebral conjunctiva. VKC is, having a higher prevalence in regions with warm and dry climate [2,3]. Recurrence of the disease and exacerbation of symptoms are more commonly observed in the season of spring (ergo “vernal”) and summer. The frequent symptoms of VKC are, itching, excessive tearing, ropy mucus production, photophobia, burning, and foreign body sensation [3,4].
VKC is characterized by an intense inflammation of eosinophils and Th2-type lymphocytes. Many cytokines, chemokines, mediators, and proteases, such as tryptase, chymase, and metalloproteases (MMPs) have been found to be over-expressed in tears and tissues of patients affected by VKC [5].
Acute phase reaction is body’s response in situations such as tissue injury, trauma, infections etc. that disrupts the normal homeostasis of the body. It’s a pathophysiological security system, associated with inflammation, important for body’s response against tissue injury and is marked by changes in the level of certain plasma proteins, known as acute phase proteins [6,7].
Alpha-1 antitrypsin (AAT) is the archetype representative of serine proteinase inhibitor (SERPIN) superfamily. This glycoprotein secreted by liver cells is considered an acute-phase reactant, since its plasma levels increase during host response to inflammation/tissue injury [8,9]. AAT is a small 52 kDa glycoprotein composed of a single polypeptide chain of 394 amino acids [10], with glycan groups attached by N-glycosidic linkage to the side-chain amide group of three asparagine residues at 46th, 83rd and 247th position [11]. Besides inhibiting trypsin, as suggested by its name [9], AAT also inhibits some other proteolytic enzymes such as neutrophil elastase, proteinase-3, cathepsin-G [12] and chymase [13]. AAT is synthesized mainly by liver, and also by macrophages, and epithelial cells of the intestine and pulmonary tract [14]. A severe deficiency of AAT creates an imbalance between proteinases and inhibitors, clinically manifesting as inflammatory pathologic events.
A few studies have been done in which the serum concentrations of AAT in VKC patients have been measured and were found to be elevated [15]. The purpose of the present study was to evaluate the serum AAT levels in VKC, and to establish any possible correlation of it with the severity of disease so that a prognostic criterion can be established.
Materials and Methods
Study Design
The study consisted of 50 patients of VKC (36 men and 14 women) with a mean age of 17.5 ± 5.2 years attending the ophthalmology OPD of JN Medical College, Aligarh, India. The control group consisted of 40 healthy, age matched subjects, 25 men and 15 women, recruited from the institution. Smokers and pregnant females were excluded from the study. The study was duly approved by the Board of Studies/Institutional Ethical Committee and a valid and informed consent was obtained from all the subjects (including both case and control).
Diagnosis of VKC was based on clinical history and evaluation of signs and symptoms. Patients included in the study were in an active inflammatory phase of the disease with active limbal infiltrates and were free of topical antihistamines and mast cell stabilizers for at least 3 days, topical corticosteroids for at least 7 days, and systemic antiallergic treatment for at least 2 week at the time of first presentations.
Blood samples for the evaluation of serum AAT levels were collected at the first OPD visit of the patients and thereafter 3 weeks post treatment with topical steroids, mast cell stabilizers and anti-histaminics. The pre and post treatment serum AAT levels were compared with each other and with that of serum AAT levels of the healthy controls. To analyse the serum AAT levels in a broader level the subjects were categorized into sub-group based on sex (males and females), age (≤15 years, 16-20 years, 21-25 years, and >25 years) and the severity of disease (mild/moderate and severe VKC patients) [Table/Fig-1].
Distribution of control and study subjects into different categories; Number of VKC patients showing increased AAT levels
| Categories | Number of subjects in | Number of VKC patients with increased AAT levels |
|---|
| Control group | Study (VKC) group | Before treatment | After treatment |
|---|
| Whole group | 40 | 50 | 34 | 9 |
| Sex | Males | 25 | 36 | 25 | 6 |
| Females | 15 | 14 | 9 | 3 |
| Age | ≤ 15 years | 9 | 16 | 12 | 3 |
| 16-20 years | 16 | 22 | 15 | 4 |
| 21-25 years | 11 | 7 | 4 | 1 |
| >25 years | 4 | 5 | 3 | 1 |
| Severity of cases | Mild/Moderate | - | 44 | 28 | 4 |
| Severe | - | 6 | 6 | 5 |
Blood collection and biochemical assay of serum AAT levels: 5 ml of blood sample was drawn under aseptic condition from the peripheral vein of the subjects. It was centrifuged at 3000 rpm for fifteen minutes. The serum thus obtained was subjected to biochemical evaluation.
Serum trypsin inhibitory activity and serum AAT levels were evaluated according to the procedure of Waheed and Salahuddin and Arjumand Sultan Warsy [16,17]. A standard solution of trypsin was allowed to react with N-a-benzoyl-DL-arginine-p-nitroanilide (BAPNA) resulting in the formation of p-nitroanilide which gives yellow colour. The intensity of colour was measured at 410 nm using Hitachi’s U-2910 double beam spectrophotometer and was used for checking the activity of trypsin in the presence and absence of serum. This provided the percentage inhibition of trypsin by the sera.
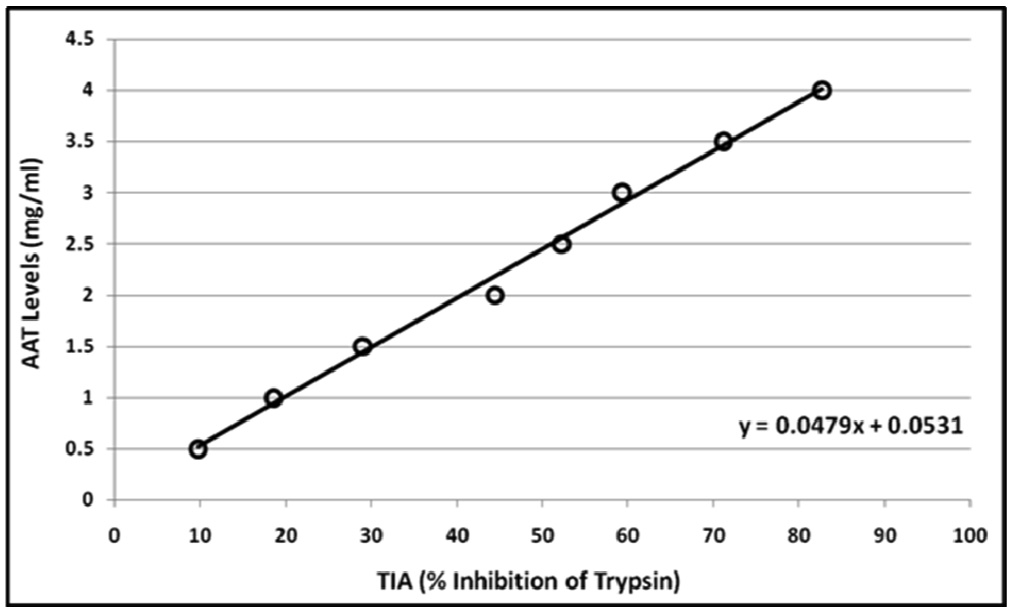
The percent inhibition of trypsin was also calculated using different concentrations of commercial alpha-1 antitrypsin solution (instead of sera), and a graph of “alpha-1 antitrypsin concentration” versus “% inhibition of trypsin” was plotted [Table/Fig-2]. The linear curve obtained by least square analysis was found to fit the equation which was used to calculate the concentration of alpha-1 antitrypsin in the serum:
Calibration curve for the estimation of serum AAT concentration using standard solution of human plasma AAT

Statistical Analysis
All data were expressed as mean ± SD. The data from patients and controls were compared using unpaired Student’s t-test whereas the pre and post treatment serum AAT levels were compared using the paired Student’s t-test. Statistical Package for Social Science (SPSS) version 20.0 was used for statistical analysis. p-value of less than 0.05 was considered to indicate statistical significance.
Results
[Table/Fig-1] shows the distribution profile of VKC patients and controls.
Serum AAT levels in patients with acute VKC: Patients in the acute stage of the disease showed significantly elevated serum AAT levels, irrespective of their age and sex and the severity of disease [Table/Fig-3]
Degree of significance as seen after statistically analysing the serum AAT levels of the VKC patients, as compared to respective controls (by applying unpaired t-test)
| Group/sub-groups | AAT level (mg/ml) of | p-value (unpaired t-test) |
|---|
| Patients | Respective Controls |
| VKC patients (acute disease) | 2.80 ± 0.42 | 2.31 ± 0.21 | < 0.01 |
| Males | 2.82 ± 0.42 | 2.34 ± 0.22 | < 0.01 |
| Females | 2.77 ± 0.40 | 2.26 ± 0.18 | < 0.01 |
| ≤15 years | 2.83 ± 0.42 | 2.37 ± 0.10 | < 0.01 |
| 16-20 years | 2.85 ± 0.45 | 2.27 ± 0.22 | < 0.01 |
| 21-25 years | 2.80 ± 0.35 | 2.28 ± 0.25 | < 0.01 |
| >25 years | 2.74 ± 0.29 | 2.40 ± 0.19 | < 0.03 |
| Mild/Moderate | 2.76 ± 0.41 | 2.31 ± 0.21 | < 0.01 |
| Severe | 3.09 ± 0.25 | 2.31 ± 0.21 | < 0.001 |
| VKC patients (after treatment) | 2.48 ± 0.26 | 2.31 ± 0.21 | > 0.08 |
| Males | 2.51 ± 0.28 | 2.34 ± 0.22 | > 0.08 |
| Females | 2.44 ± 0.23 | 2.26 ± 0.18 | > 0.07 |
| ≤15 years | 2.52 ± 0.29 | 2.37 ± 0.10 | > 0.08 |
| 16-20 years | 2.46 ± 0.25 | 2.27 ± 0.22 | > 0.07 |
| 21-25 years | 2.43 ± 0.21 | 2.28 ± 0.25 | > 0.08 |
| >25 years | 2.49 ± 0.17 | 2.40 ± 0.19 | > 0.10 |
| Mild/Moderate | 2.43 ± 0.24 | 2.31 ± 0.21 | > 0.10 |
| Severe | 2.88 ± 0.21 | 2.31 ± 0.21 | < 0.01 |
Serum AAT levels in VKC patients 3 weeks post-treatment: The post treatment serum AAT levels didn’t show significant elevation when compared to controls [Table/Fig-3].
The VKC patients displayed a significant fall in their AAT levels post-treatment. The only exceptions were the severe cases of VKC who didn’t show a significant fall in their AAT levels after treatment; instead they exhibited significantly elevated levels (as compared to controls) even after treatment [Table/Fig-3,4].
Significance values obtained after statistically analysing the serum AAT levels of the VKC patients, before and after treatment (by applying paired t-test)
| Group/Sub-group | AAT level (mg/ml) of | p-value (paired t-test) |
|---|
| VKC patients (acute stage) | VKC patients (after treatment) |
| Whole group | 2.80 ± 0.42 | 2.48 ± 0.26 | < 0.03 |
| Males | 2.82 ± 0.42 | 2.51 ± 0.28 | < 0.03 |
| Females | 2.77 ± 0.40 | 2.44 ± 0.23 | < 0.03 |
| ≤15 years | 2.83 ± 0.42 | 2.52 ± 0.29 | < 0.03 |
| 16-20 years | 2.85 ± 0.45 | 2.46 ± 0.25 | < 0.02 |
| 21-25 years | 2.80 ± 0.35 | 2.43 ± 0.21 | < 0.02 |
| >25 years | 2.74 ± 0.29 | 2.49 ± 0.17 | < 0.03 |
| Mild/Moderate | 2.76 ± 0.41 | 2.43 ± 0.24 | < 0.03 |
| Severe | 3.09 ± 0.25 | 2.88 ± 0.21 | > 0.07 |
Discussion
AAT shows a rapid rise in plasma concentration in response to inflammation and injury and is therefore an appreciated acute phase protein [18]. Serum levels and activity of AAT have been shown to be increased as a part of acute phase response towards tissue injury and/or inflammatory conditions such as bacterial and viral infections, rheumatoid arthritis, hepatitis, carcinomas, vasculitis, and uveitis [19,20]. VKC is a chronic ocular condition represented by profound inflammation of the ocular surface and elevated eosinophils and leukocyte activation markers [5]. The study shows significantly raised serum AAT levels in the acute phase of the disease, irrespective of the age and sex of the VKC patients; as such serum AAT levels can be utilized as a biochemical marker to establish the diagnosis of the VKC patients.
Chymase, a chymotrypsin like protease, secreted from mast cells is found to be elevated in VKC patients [5]. Since, chymase is kept in control by AAT [21], it is quite possible that overexpression of chymase triggers the up-regulation of AAT in VKC patients. Chymase converts angiotensin-I to angiotensin-II [22] and also activates some endogenous peptides like endothelin, stem cell factor and interleukin-1β (IL-1β) [23], IL-1, IL-6, TNF-α and lipopolysaccharides have shown to up-regulate the synthesis of AAT [24]. IL-1 may therefore account for the increased antitryptic activity in the serum of VKC patients.
After 3 weeks of treatment almost all the patients displayed impressive improvement in their condition with no signs of inflammation, which was also accompanied with fall in the serum AAT levels. Serum AAT level of the VKC patients after treatment was slightly higher than the control subjects but the difference was not significant (p>0.08), but it was significantly lower than the AAT levels of the patients before treatment (p<0.03), suggesting that the decline in the plasma AAT concentrations might be a result of the reduction in inflammation. The decrease in the serum AAT levels was significant not only for the whole group but also for all the sub-groups of VKC patients (age- and sex- groups), leading to the hypothesis that it can be used as an analytical marker to check the response to treatment in VKC patients.
The study attempts to establish a correlation between the severity of disease and serum level of AAT. Although, the patients who presented with mild to moderate signs/symptoms of the disease (n=44) showed significantly elevated AAT levels, patients with severe disease (n=6) displayed even higher serum AAT levels. Post-treatment, patients with mild/moderate disease showed complete recovery along with a significant decline in their AAT levels approaching that of controls. On the other hand, the 6 patients who presented with severe form of the disease showed little improvement and post-treatment their serum AAT level was still significantly higher than the control subjects. These observations lead to the assumption that the serum AAT levels may be affected by the severity of the disease. The increasing severity and greater inflammatory damage may result in higher AAT concentrations in the serum and therefore the serum AAT levels might be helpful in determining the prognosis of the disease.
Conclusion
Although, the data of severe VKC patients is not sufficient enough, but with further studies and more promising data this hypothesis can be proved. Not only the serum AAT levels might be of help in establishing the diagnosis of the disease but it can also be considered in monitoring the response to treatment and determining the prognosis of the patients at any given point.
[1]. Stahl JL, Barney NP, Ocular allergic diseaseCurr Opin Allergy Clin Immunol 2004 4(5):455-9. [Google Scholar]
[2]. Kawuma M, The clinical picture of vernal kerato-conjunctivitis in UgandaCommunity Eye Health 2001 14(40):66-7. [Google Scholar]
[3]. Bonini S, Vernal keratoconjunctivitis revisited: a case series of 195 patients with long-term followupOphthalmology 2000 107(6):1157-63. [Google Scholar]
[4]. Allansmith MR, Ross RN, Ocular allergyClin Allergy 1988 18(1):1-13. [Google Scholar]
[5]. Leonardi A, Vernal keratoconjunctivitis: pathogenesis and treatmentProg Retin Eye Res 2002 21(3):319-39. [Google Scholar]
[6]. Gabay C, Kushner I, Acute-phase proteins and other systemic responses to inflammationN Engl J Med 1999 340(6):448-54. [Google Scholar]
[7]. Volanakis JE, In: Koopmann WJ, Moreland LW eds. Acute-phase Proteins in Rheumatic Disease 2005 PhiladelphiaLippincott Williams & Wilkins:15 [Google Scholar]
[8]. Morgan K, Kalsheker NA, Regulation of the serine proteinase inhibitor (SERPIN) gene alpha 1-antitrypsin: a paradigm for other SERPINsInt J Biochem Cell Biol 1997 29(12):1501-11. [Google Scholar]
[9]. Schultze HE, Heide K, Haupt H, alpha1-Antitrypsin from human serumKlin Wochenschr 1962 40:427-9. [Google Scholar]
[10]. Lomas DA, Parfrey H, Alpha1-antitrypsin deficiency. 4: Molecular pathophysiologyThorax 2004 59(6):529-35. [Google Scholar]
[11]. Carrell RW, Structure and variation of human alpha 1-antitrypsinNature 1982 298(5872):329-34. [Google Scholar]
[12]. Korkmaz B, Neutrophil elastase, proteinase 3, and cathepsin G as therapeutic targets in human diseasesPharmacol Rev 2010 62(4):726-59. [Google Scholar]
[13]. Chang YH, Secretomic analysis identifies alpha-1 antitrypsin (A1AT) as a required protein in cancer cell migration, invasion, and pericellular fibronectin assembly for facilitating lung colonization of lung adenocarcinoma cellsMol Cell Proteomics 2012 11(11):1320-39. [Google Scholar]
[14]. Topic A, Alpha-1-antitrypsin deficiency in Serbian adults with lung diseasesGenet Test Mol Biomarkers 2012 16(11):1282-6. [Google Scholar]
[15]. Ghavami S, Trypsin inhibitory capacity in vernal keratoconjunctivitisInvest Ophthalmol Vis Sci 2007 48(1):264-9. [Google Scholar]
[16]. Waheed A, and A. Salhuddin, Isolation and characterization of a variant of ovomucoidBiochem J 1975 147(1):139-44. [Google Scholar]
[17]. Warsy AS, Alpha-1-antitrypsin in Saudi populationJournal of Islamic Academy of Sciences 1989 2(2):85-8. [Google Scholar]
[18]. Ghavami S, Alpha-1-antitrypsin phenotypes and HLA-B27 typing in uveitis patients in southeast IranClin Biochem 2005 38(5):425-32. [Google Scholar]
[19]. Carrell RW, Lomas DA, Conformational diseaseLancet 1997 350(9071):134-8. [Google Scholar]
[20]. Hashemi M, High prevalence of alpha 1 antitrypsin phenotypes in viral hepatitis B infected patients in IranHepatol Res 2005 33(4):292-7. [Google Scholar]
[21]. Wasse H, Increased plasma chymase concentration and mast cell chymase expression in venous neointimal lesions of patients with CKD and ESRDSemin Dial 2011 24(6):688-93. [Google Scholar]
[22]. Miyazaki M, Takai S, Tissue angiotensin II generating system by angiotensin-converting enzyme and chymaseJ Pharmacol Sci 2006 100(5):391-7. [Google Scholar]
[23]. Thorpe M, Extended cleavage specificity of the mast cell chymase from the crab-eating macaque (Macaca fascicularis): an interesting animal model for the analysis of the function of the human mast cell chymaseInt Immunol 2012 24(12):771-82. [Google Scholar]
[24]. Knoell DL, Alpha 1-antitrypsin and protease complexation is induced by lipopolysaccharide, interleukin-1beta, and tumor necrosis factor-alpha in monocytesAm J Respir Crit Care Med 1998 157(1):246-55. [Google Scholar]